Towards Correlative Raman Spectroscopy–STEM Investigations Performed on a Magnesium–Silver Alloy FIB Lamella
Abstract
:1. Introduction
2. Materials and Methods
3. Results
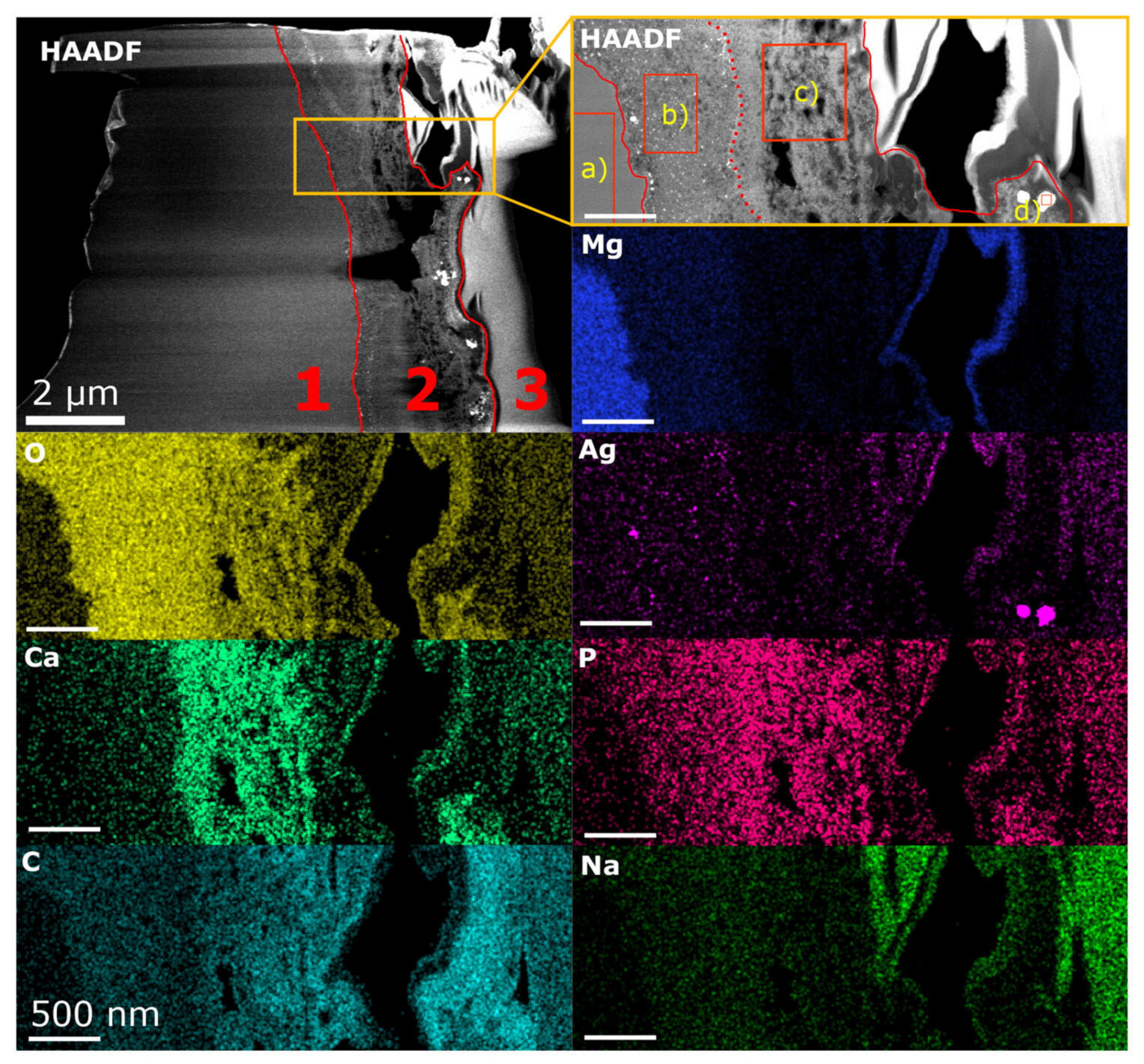
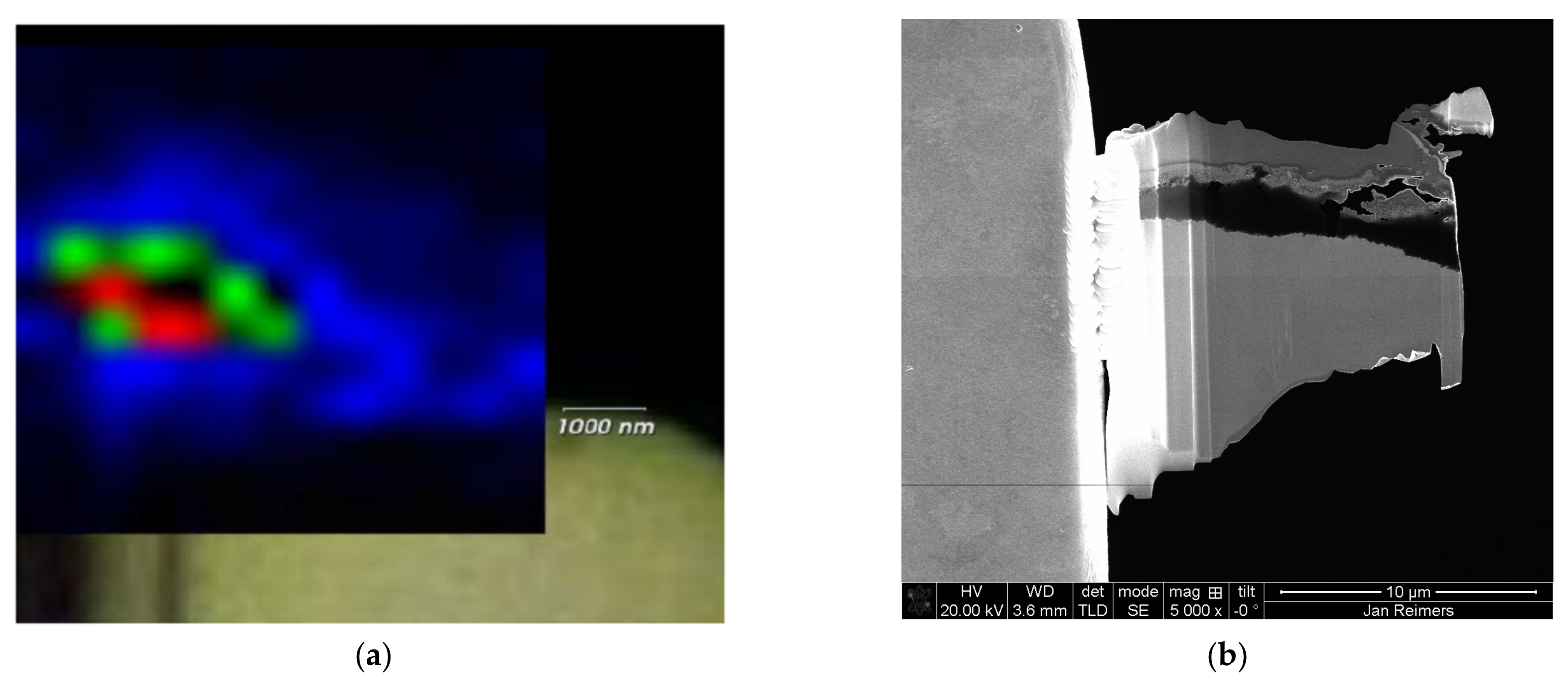
3.1. Microstructural Analysis Using HAADF STEM and EDX
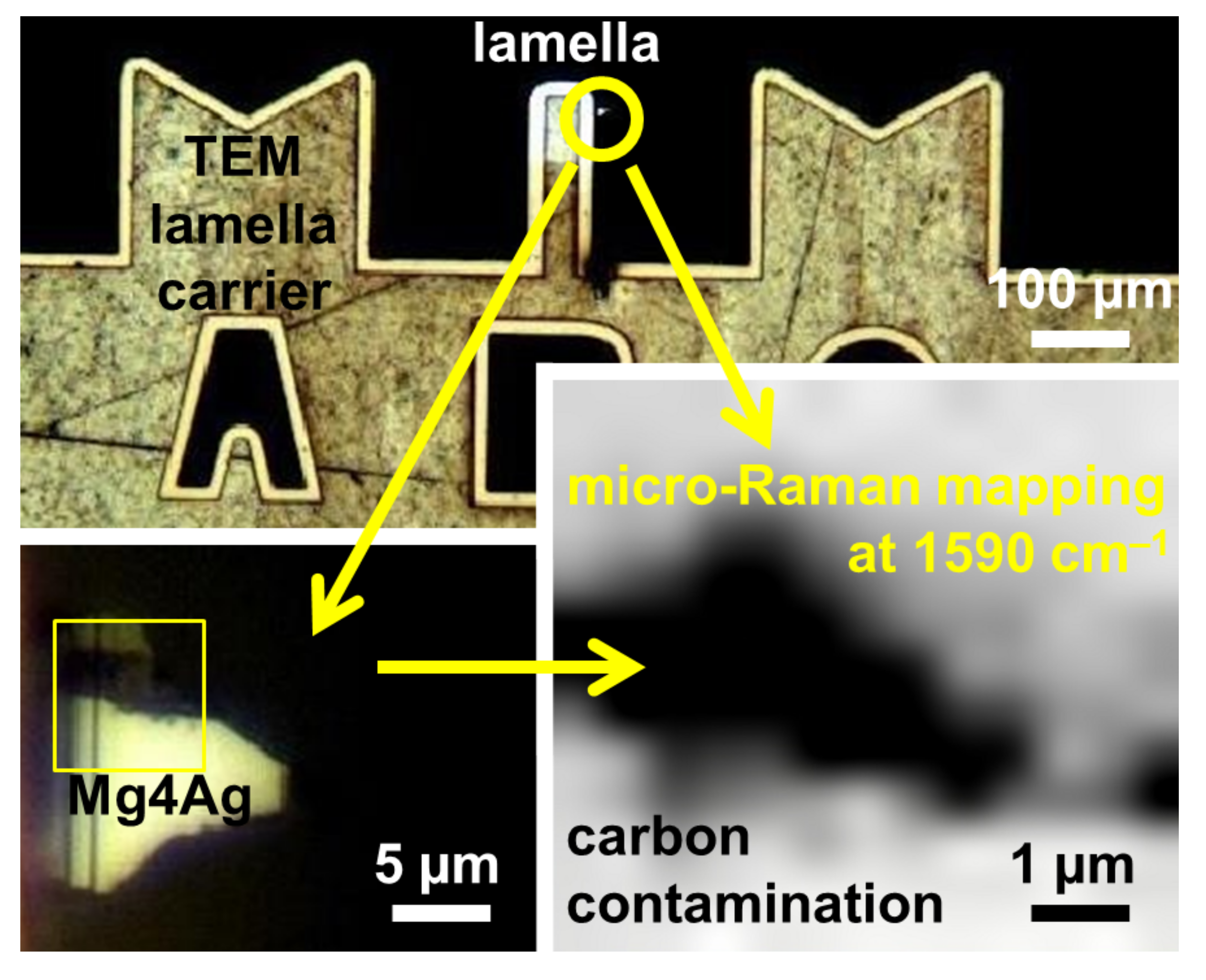
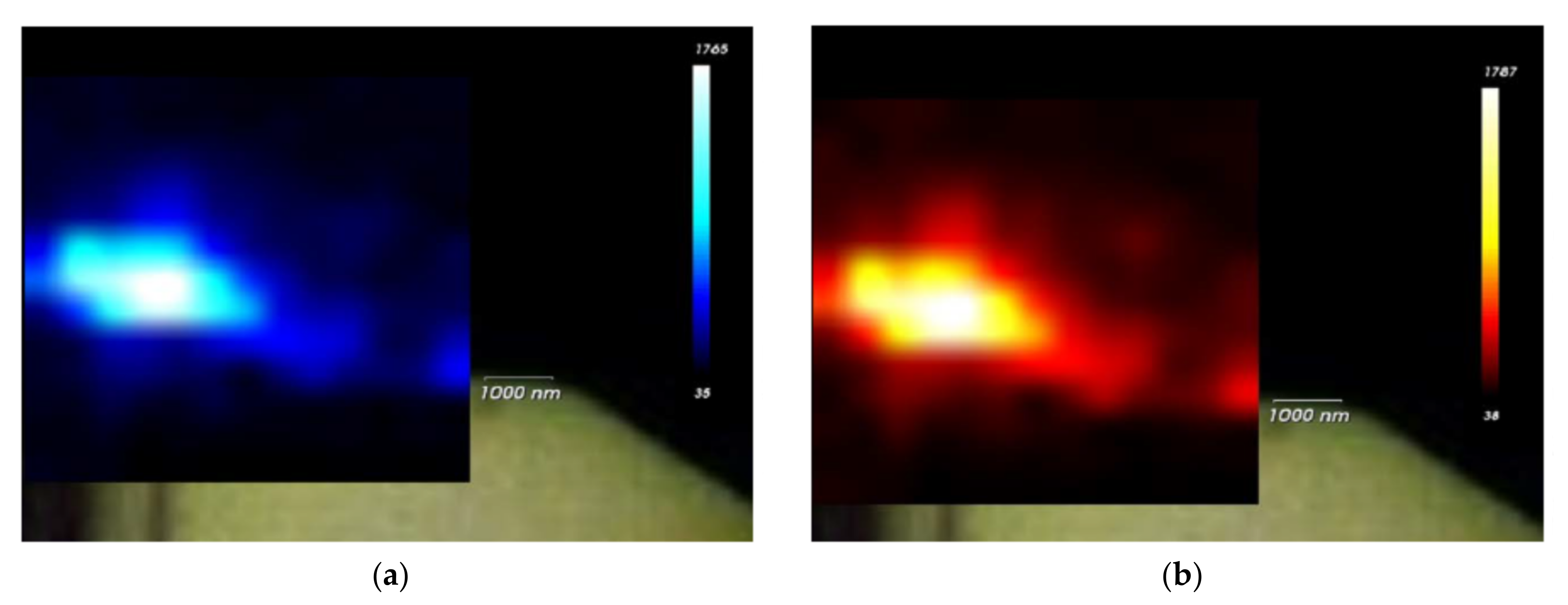
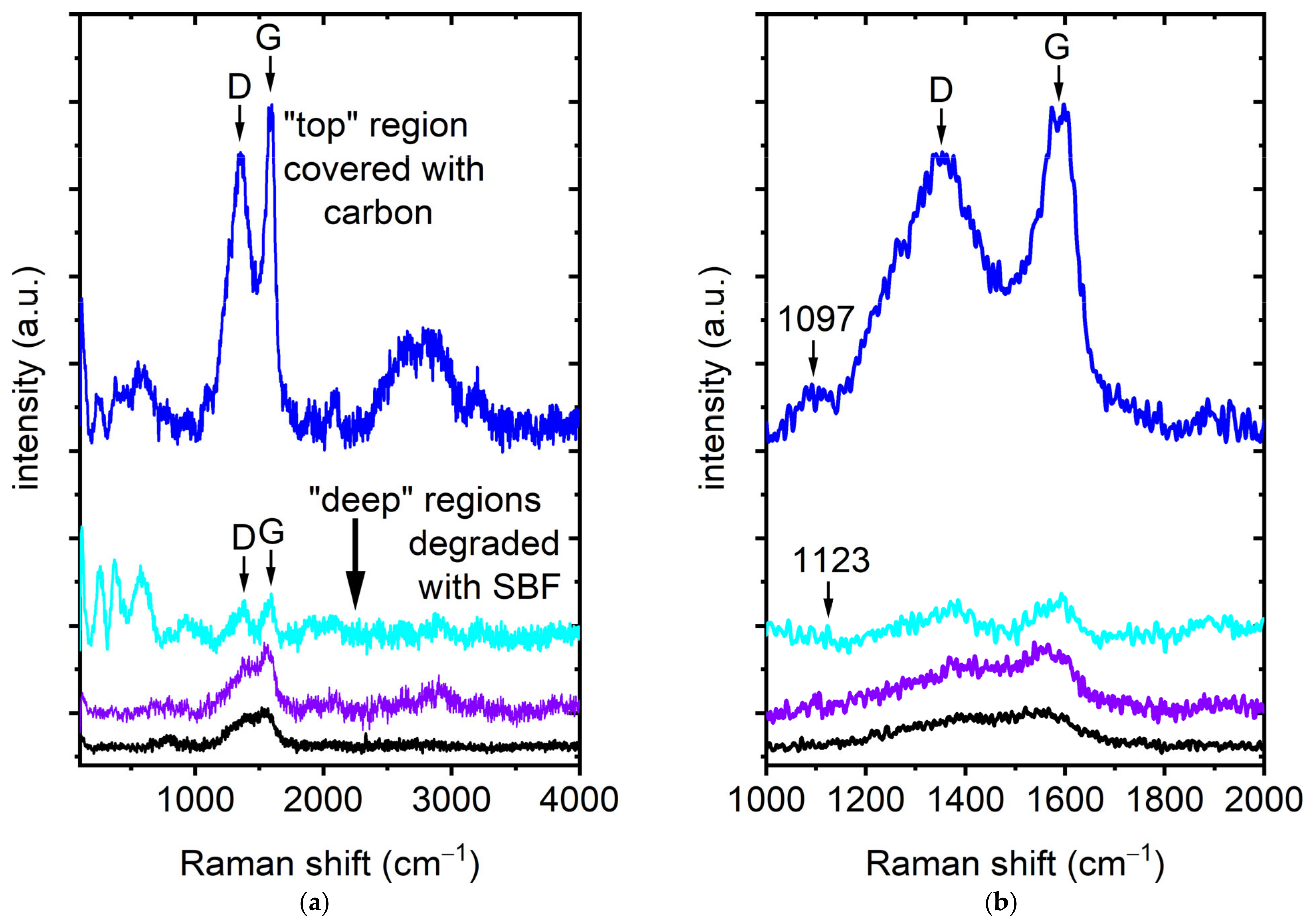
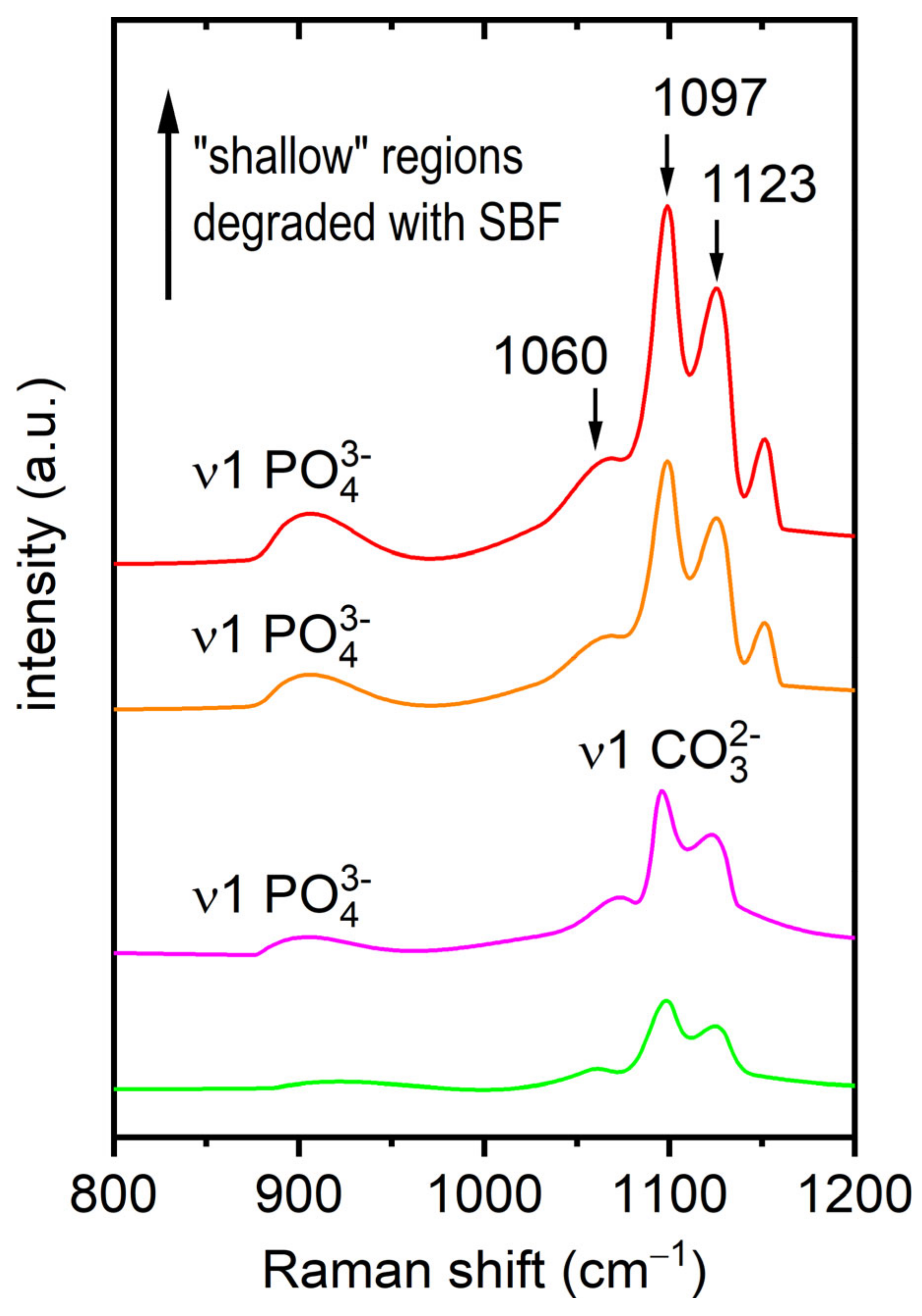
3.2. Raman Spectroscopy
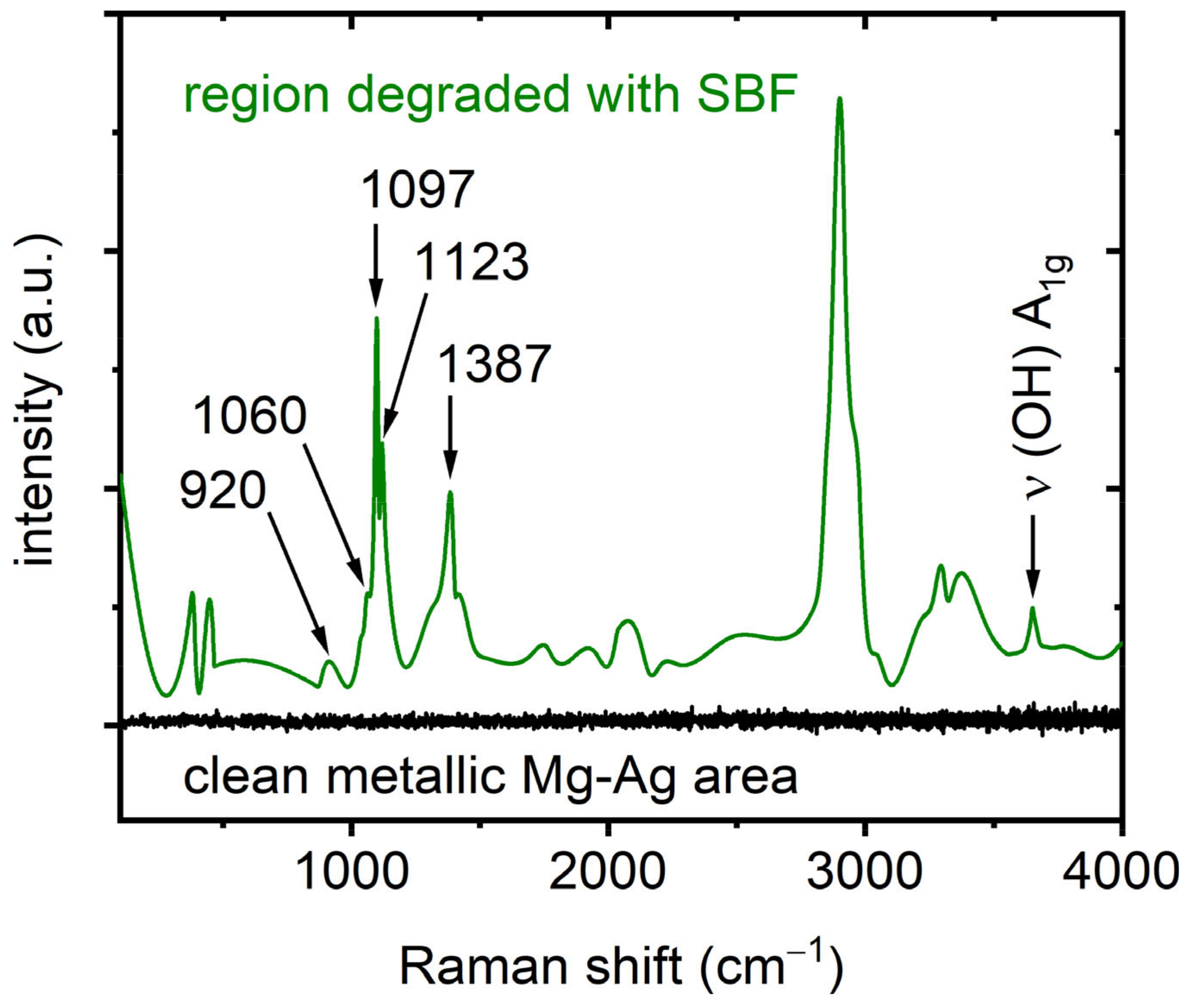
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Pinc, J.; Školáková, A.; Hybášek, V.; Msallamová, Š.; Veřtát, P.; Ashcheulov, P.; Vondráček, M.; Duchoň, J.; McCarroll, I.; Hývl, M.; et al. A detailed mechanism of degradation behaviour of biodegradable as-ECAPed Zn-0.8Mg-0.2Sr with emphasis on localized corrosion attack. Bioact. Mater. 2023, 27, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.M.; Yang, J.; Aota, L.S.; Woods, E.; Zhou, X.; Neugebauer, J.; Todorova, M.; McCarroll, I.; Gault, B. Quasi-“In Situ” Analysis of the Reactive Liquid-Solid Interface during Magnesium Corrosion Using Cryo-Atom Probe Tomography. Adv. Mater. 2024, 36, 2401735. [Google Scholar] [CrossRef] [PubMed]
- Nasr Azadani, M.; Zahedi, A.; Bowoto, O.K.; Oladapo, B.I. A review of current challenges and prospects of magnesium and its alloy for bone implant applications. Prog. Biomater. 2022, 11, 1–26. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl. Catal. B Environ. 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Wang, J.; Meng, L.; Xie, W.; Ji, C.; Wang, R.; Zhang, P.; Jin, L.; Sheng, L.; Zheng, Y. Corrosion and in vitro cytocompatibility investigation on the designed Mg-Zn-Ag metallic glasses for biomedical application. J. Magnes. Alloy. 2024, 12, 1566–1580. [Google Scholar] [CrossRef]
- Wang, C.; Qian, K.; Wu, Y.; Mei, D.; Chu, C.; Xue, F.; Bai, J.; Zheludkevich, M.L.; Lamaka, S.V. Consistent high rate oxygen reduction reaction during corrosion of Mg-Ag Alloy. Corros. Sci. 2024, 229, 111893. [Google Scholar] [CrossRef]
- Asgari, M.; Hang, R.; Wang, C.; Yu, Z.; Li, Z.; Xiao, Y. Biodegradable Metallic Wires in Dental and Orthopedic Applications: A Review. Metals 2018, 8, 212. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A review on magnesium alloys for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu EP, S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium degradation under physiological conditions–Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Ghali, E.; Dietzel, W.; Kainer, K.-U. General and Localized Corrosion of Magnesium Alloys: A Critical Review. J. Mater. Eng. Perform. 2004, 13, 7–23. [Google Scholar] [CrossRef]
- Wang, J.; Jang, Y.; Wan, G.; Giridharan, V.; Song, G.-L.; Xu, Z.; Koo, Y.; Qi, P.; Sankar, J.; Huang, N.; et al. Flow-induced corrosion of absorbable magnesium alloy: In-situ and real-time electrochemical study. Corros. Sci. 2016, 104, 277–289. [Google Scholar] [CrossRef]
- Bär, F.; Berger, L.; Jauer, L.; Kurtuldu, G.; Schäublin, R.; Schleifenbaum, J.H.; Löffler, J.F. Laser additive manufacturing of biodegradable magnesium alloy WE43: A detailed microstructure analysis. Acta Biomater. 2019, 98, 36–49. [Google Scholar] [CrossRef]
- Cha, P.-R.; Han, H.-S.; Yang, G.-F.; Kim, Y.-C.; Hong, K.-H.; Lee, S.-C.; Jung, J.-Y.; Ahn, J.-P.; Kim, Y.-Y.; Cho, S.-Y.; et al. Biodegradability engineering of biodegradable Mg alloys: Tailoring the electrochemical properties and microstructure of constituent phases. Sci. Rep. 2013, 3, 2367. [Google Scholar] [CrossRef]
- Reimers, J.; Trinh, H.C.; Wiese, B.; Meyer, S.; Brehling, J.; Flenner, S.; Hagemann, J.; Kruth, M.; Kibkalo, L.; Ćwieka, H.; et al. Development of a Bioreactor-Coupled Flow-Cell Setup for 3D In Situ Nanotomography of Mg Alloy Biodegradation. ACS Appl. Mater. Interfaces 2023, 15, 35600–35610. [Google Scholar] [CrossRef]
- Timmermans, F.J.; Liszka, B.; Lenferink, A.T.M.; van Wolferen, H.A.G.M.; Otto, C. Integration of correlative Raman microscopy in a dualbeam FIB SEM. J. Raman Spectrosc. 2016, 47, 956–962. [Google Scholar] [CrossRef]
- Nasdala, L.; Akhmadaliev, S.; Artac, A.; Chanmuang, N.C.; Habler, G.; Lenz, C. Irradiation effects in monazite–(Ce) and zircon: Raman and photoluminescence study of Au-irradiated FIB foils. Phys. Chem. Miner. 2018, 45, 855–871. [Google Scholar] [CrossRef]
- Sharma, R. Experimental set up for in situ transmission electron microscopy observations of chemical processes. Micron 2012, 43, 1147–1155. [Google Scholar] [CrossRef]
- Picher, M.; Mazzucco, S.; Blankenship, S.; Sharma, R. Vibrational and optical spectroscopies integrated with environmental transmission electron microscopy. Ultramicroscopy 2015, 150, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.L.; Stach, E.A.; Arslan, I.; Crozier, P.A.; Kabius, B.C.; LaGrange, T.; Minor, A.M.; Takeda, S.; Tanase, M.; Wagner, J.B.; et al. Current status and future directions for in situ transmission electron microscopy. Ultramicroscopy 2016, 170, 86–95. [Google Scholar] [CrossRef]
- Allen, F.I.; Kim, E.; Andresen, N.C.; Grigoropoulos, C.P.; Minor, A.M. In situ TEM Raman spectroscopy and laser-based materials modification. Ultramicroscopy 2017, 178, 33–37. [Google Scholar] [CrossRef]
- Meyer, S.; Wiese, B.; Hort, N.; Willumeit-Römer, R. Characterization of the deformation state of magnesium by electrical resistance. Scr. Mater. 2022, 215, 114712. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Kovács, A.; Schierholz, R.; Tillmann, K. FEI Titan G2 80-200 CREWLEY. J. Large-Scale Res. Facil. JLSRF 2016, 2, A43. [Google Scholar] [CrossRef]
- Mikulics, M.; Adam, R.; Chen, G.; Chakraborty, D.; Cheng, J.; Pericolo, A.; Komissarov, I.; Bürgler, D.E.; Heidtfeld, S.F.; Serafini, J.; et al. Determination of Thermal Damage Threshold in THz Photomixers Using Raman Spectroscopy. Crystals 2023, 13, 1267. [Google Scholar] [CrossRef]
- Dawson, P.; Hadfield, C.D.; Wilkinson, G.R. The polarized infra-red and Raman spectra of Mg(OH)2 and Ca(OH)2. J. Phys. Chem. Solids 1973, 34, 1217–1225. [Google Scholar] [CrossRef]
- Duffy, T.S.; Meade, C.; Fei, Y.; Mao, H.-K.; Hemley, R.J. High-pressure phase transition in brucite, Mg(OH)2. Am. Mineral. 1995, 80, 222–230. [Google Scholar] [CrossRef]
- Frost, R.L. Raman spectroscopic study of the magnesium carbonate mineral hydromagnesite (Mg5[(CO3)4(OH)2]·4H2O). J. Raman Spectrosc. 2011, 42, 1690–1694. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, Z.; Leng, Y.; Wang, H.; Song, F.; Meng, J. Raman characteristics of hydrocarbon and hydrocarbon inclusions. Sci. China Ser. D Earth Sci. 2007, 50, 1171–1178. [Google Scholar] [CrossRef]
- Alajtal, A.I.; Edwards HG, M.; Elbagerma, M.A.; Scowen, I.J. The effect of laser wavelength on the Raman Spectra of phenanthrene, chrysene, and tetracene: Implications for extra-terrestrial detection of polyaromatic hydrocarbons. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A.; Lucchese, M.M.; Stavale, F.; Martins Ferreira, E.H.; Moutinho MV, O.; Capaz, R.B.; Achete, C.A. Raman study of ion-induced defects in N -layer graphene. J. Phys. Condens. Matter 2010, 22, 334204. [Google Scholar] [CrossRef] [PubMed]
- Si, G.; Liu, J.; Li, Z.; Ning, Z.; Fang, Y. Quartz Tube Enhanced Raman Scattering Spectroscopy. Front. Phys. 2022, 10, 930007. [Google Scholar] [CrossRef]
- Oliver, B.G.; Davis, A.R. Vibrational Spectroscopic Studies of Aqueous Alkali Metal Bicarbonate and Carbonate Solutions. Can. J. Chem. 1973, 51, 698–702. [Google Scholar] [CrossRef]
- Dekermenjian, M.; Ruediger, A.P.; Merlen, A. Raman spectroscopy investigation of magnesium oxide nanoparticles. RSC Adv. 2023, 13, 26683–26689. [Google Scholar] [CrossRef]
- Schmid, T.; Dariz, P. Shedding light onto the spectra of lime: Raman and luminescence bands of CaO, Ca(OH)2 and CaCO3. J. Raman Spectrosc. 2015, 46, 141–146. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J.; Xi, Y. A Raman spectroscopic study of the mono-hydrogen phosphate mineral dorfmanite Na2(PO3OH)·2H2O and in comparison with brushite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 132–136. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Gile, M.; Priebe, M.; Slominska, H.; Boll, B.; Wiese, B.; Würger, T.; Willumeit-Römer, R.; Meißner, R.H. Exploring key ionic interactions for magnesium degradation in simulated body fluid—A data-driven approach. Corros. Sci. 2021, 182, 109272. [Google Scholar] [CrossRef]
- Ahmad Agha, N.; Willumeit-Römer, R.; Laipple, D.; Luthringer, B.; Feyerabend, F. The Degradation Interface of Magnesium Based Alloys in Direct Contact with Human Primary Osteoblast Cells. PLoS ONE 2016, 11, e0157874. [Google Scholar] [CrossRef]
- Gonzalez, J.; Lamaka, S.V.; Mei, D.; Scharnagl, N.; Feyerabend, F.; Zheludkevich, M.L.; Willumeit-Römer, R. Mg Biodegradation Mechanism Deduced from the Local Surface Environment under Simulated Physiological Conditions. Adv. Healthc. Mater. 2021, 10, 2100053. [Google Scholar] [CrossRef] [PubMed]
- Gnedenkov, A.S.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Imshinetskiy, I.M.; Zavidnaya, A.G.; Zheludkevich, M.L.; Gnedenkov, S.V. Electrochemical behaviour of the MA8 Mg alloy in minimum essential medium. Corros. Sci. 2020, 168, 108552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reimers, J.; Mikulics, M.; Lipinska-Chwalek, M.; Zeller-Plumhoff, B.; Kibkalo, L.; Kruth, M.; Willumeit-Römer, R.; Mayer, J.; Hardtdegen, H.H. Towards Correlative Raman Spectroscopy–STEM Investigations Performed on a Magnesium–Silver Alloy FIB Lamella. Nanomaterials 2025, 15, 430. https://doi.org/10.3390/nano15060430
Reimers J, Mikulics M, Lipinska-Chwalek M, Zeller-Plumhoff B, Kibkalo L, Kruth M, Willumeit-Römer R, Mayer J, Hardtdegen HH. Towards Correlative Raman Spectroscopy–STEM Investigations Performed on a Magnesium–Silver Alloy FIB Lamella. Nanomaterials. 2025; 15(6):430. https://doi.org/10.3390/nano15060430
Chicago/Turabian StyleReimers, Jan, Martin Mikulics, Marta Lipinska-Chwalek, Berit Zeller-Plumhoff, Lidia Kibkalo, Maximilian Kruth, Regine Willumeit-Römer, Joachim Mayer, and Hilde Helen Hardtdegen. 2025. "Towards Correlative Raman Spectroscopy–STEM Investigations Performed on a Magnesium–Silver Alloy FIB Lamella" Nanomaterials 15, no. 6: 430. https://doi.org/10.3390/nano15060430
APA StyleReimers, J., Mikulics, M., Lipinska-Chwalek, M., Zeller-Plumhoff, B., Kibkalo, L., Kruth, M., Willumeit-Römer, R., Mayer, J., & Hardtdegen, H. H. (2025). Towards Correlative Raman Spectroscopy–STEM Investigations Performed on a Magnesium–Silver Alloy FIB Lamella. Nanomaterials, 15(6), 430. https://doi.org/10.3390/nano15060430







