Synthesis, Characterization, and Stability Study of Selenium Nanoparticles Coated with Purified Polysaccharides from Ononis natrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification of Polysaccharides Extracted from O. natrix
2.3. Synthesis of SeNPs Stabilized with Purified Polysaccharides P2 (P2-SeNPs)
2.4. Physicochemical Characterization
2.4.1. Gel Permeation Chromatography
2.4.2. Nuclear Magnetic Resonance
2.4.3. Fourier-Transform Infrared (FT-IR) and UV-Visible Spectroscopy
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Dynamic Light Scattering (DLS)
2.4.6. Transmission Electron Microscopy (TEM)
2.4.7. X-Ray Diffraction (XRD)
2.4.8. X-Ray Photoelectron Spectroscopy (XPS)
2.5. Stability Test
2.6. Antioxidant Activities
2.7. Statistical Analysis
3. Results
3.1. Purification of O. natrix Polysaccharides
3.2. Structural Characterization of Purified Polysaccharides
3.2.1. Molecular Weights
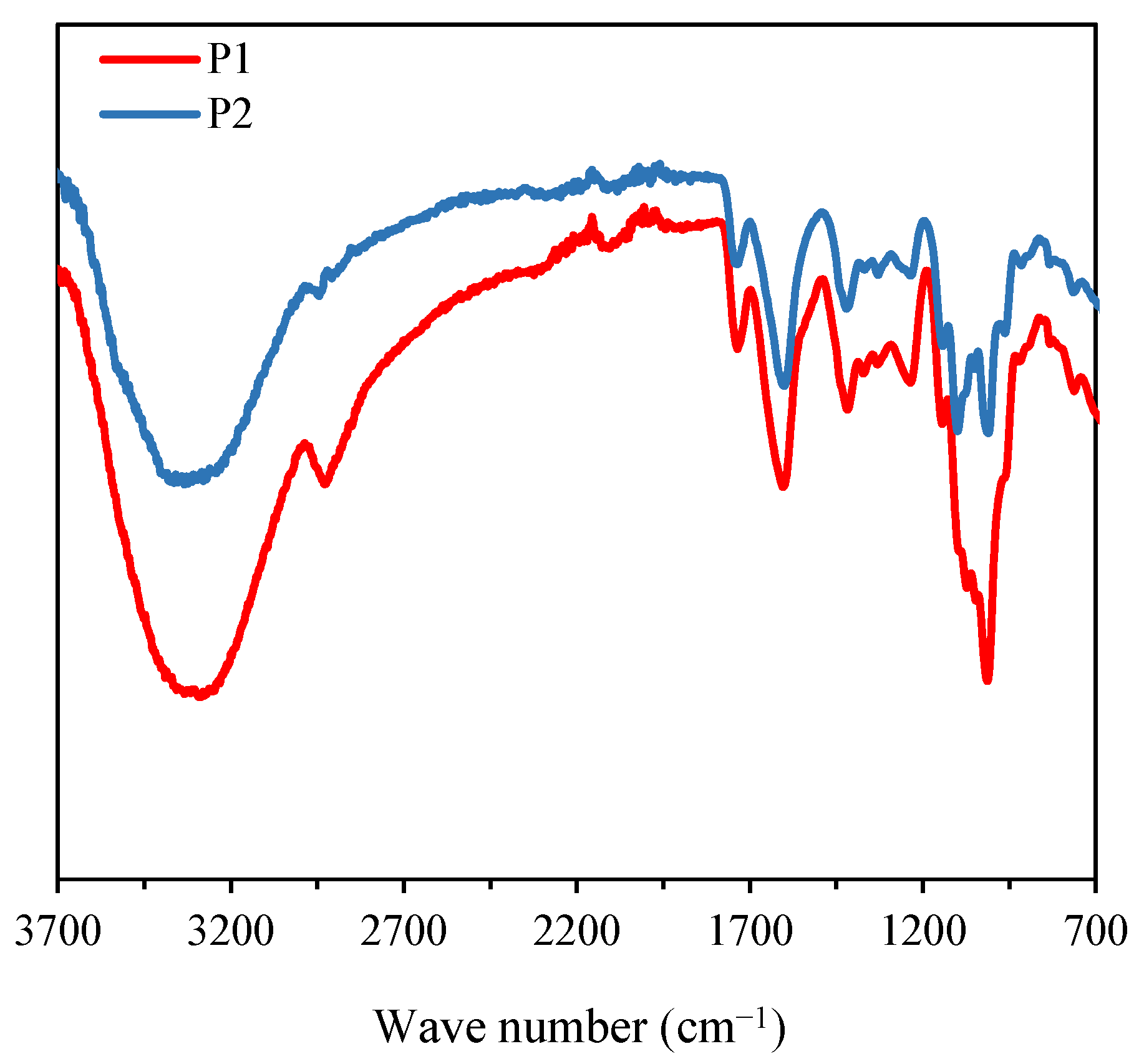
3.2.2. FT-IR Analysis
3.2.3. NMR Analysis of Purified Polysaccharides
3.2.4. Thermogravimetric Analysis of Purified Polysaccharides
3.3. Synthesis and Characterization of P2 Stabilized Selenium Nanoparticles
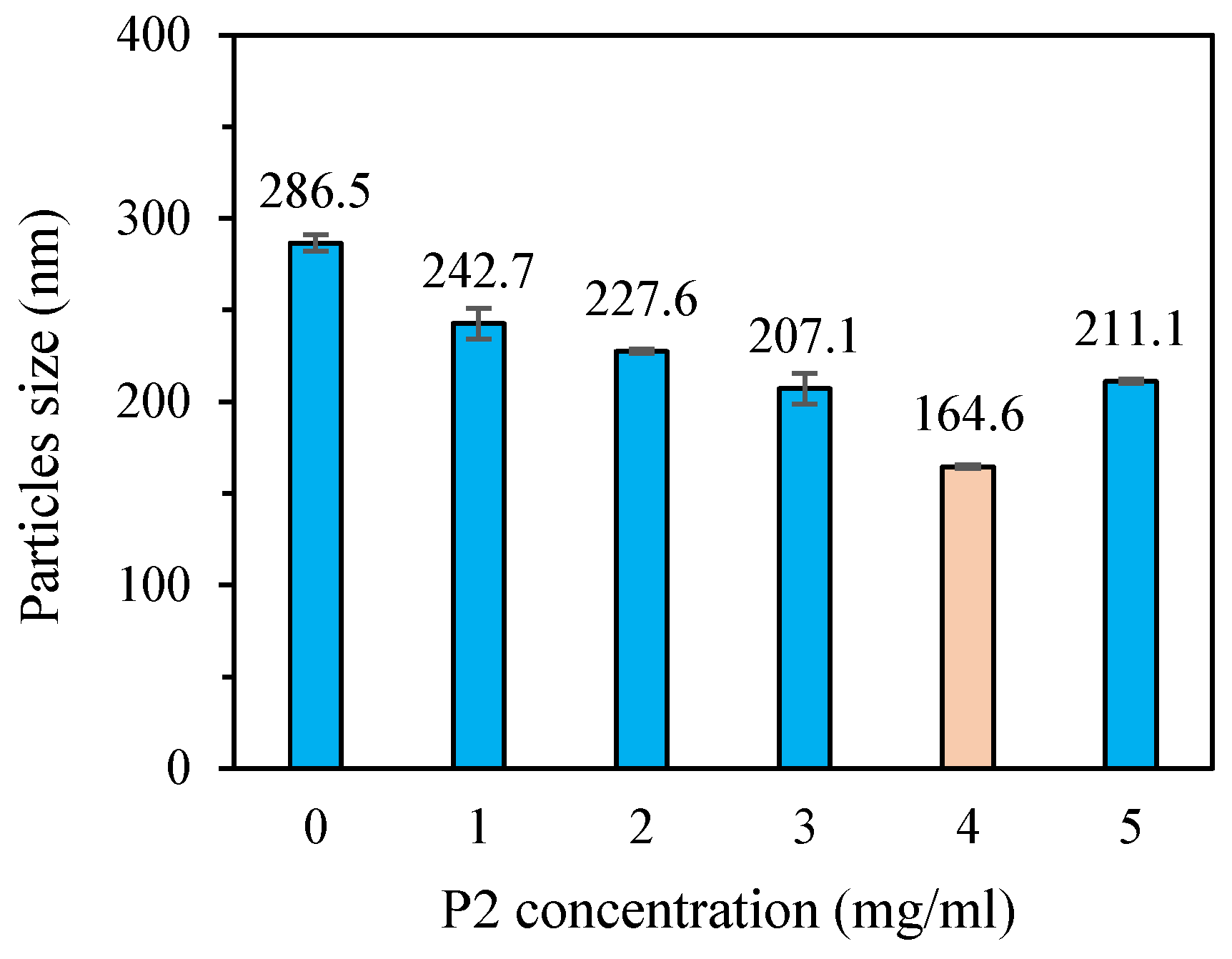
3.3.1. Particle Size and Dispersion
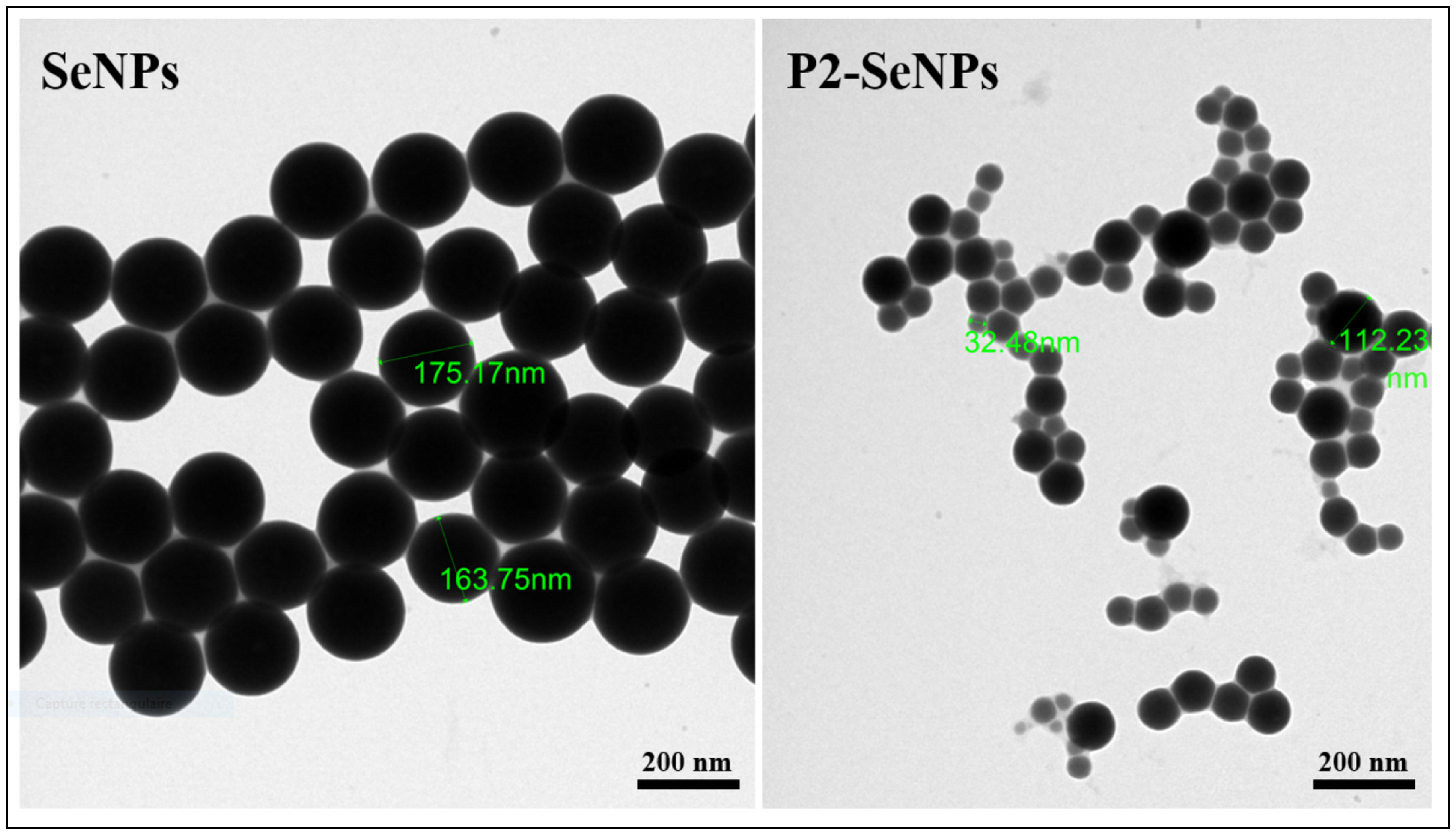
3.3.2. Morphological Evaluation
3.3.3. Interactions Between P2 and SeNPs via FT-IR Spectroscopy Analysis
3.3.4. UV-Vis Spectroscopy Analysis
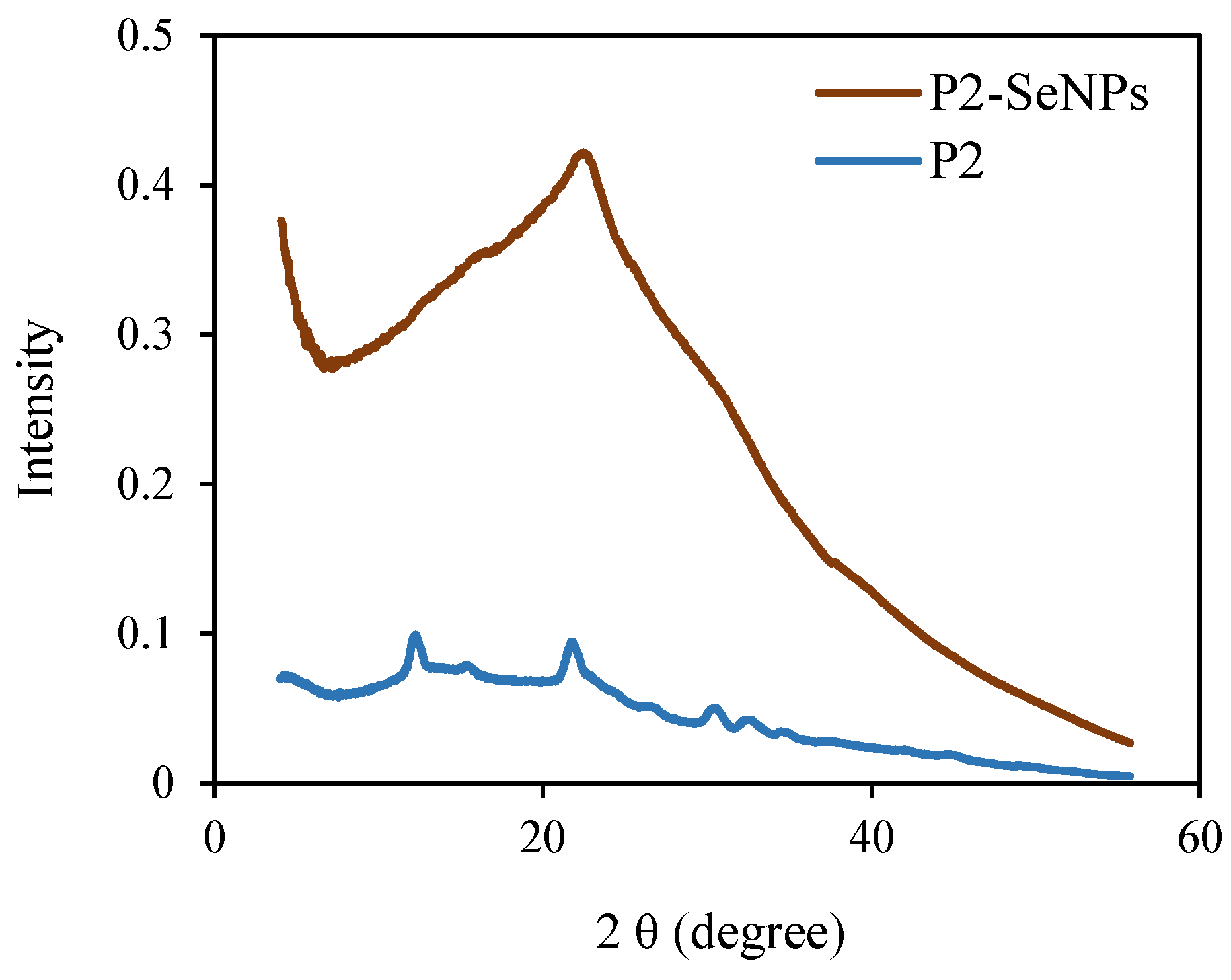
3.3.5. XRD Analysis
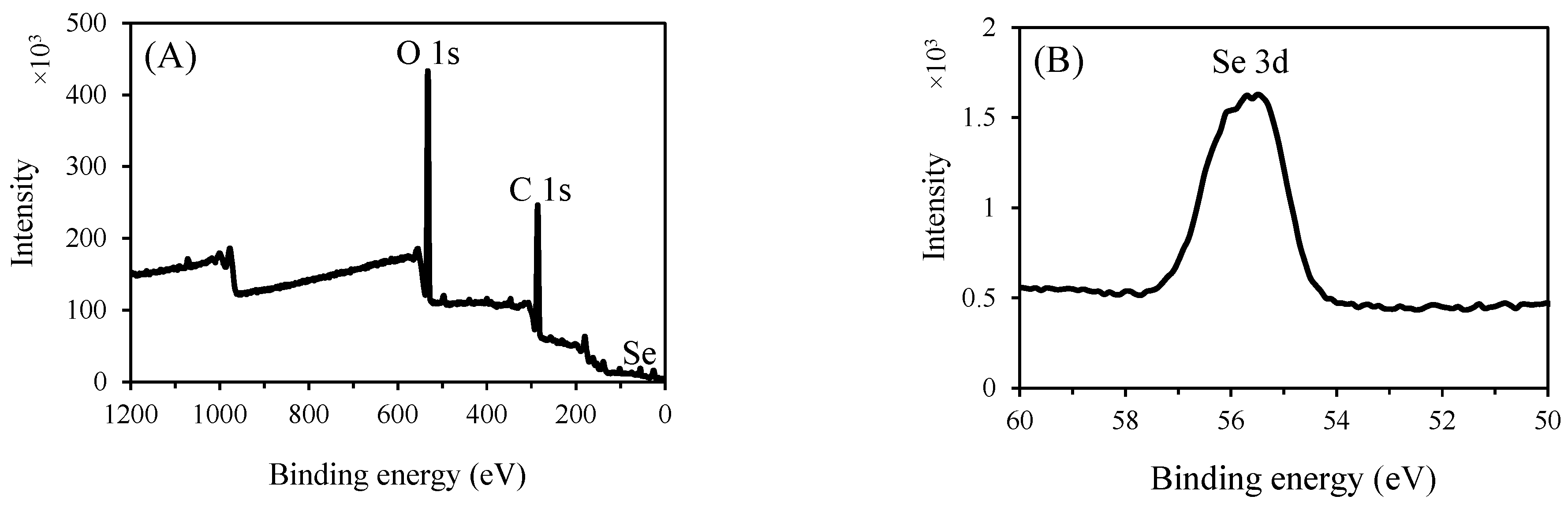
3.3.6. XPS Analysis
3.4. Stability of P2-SeNPs
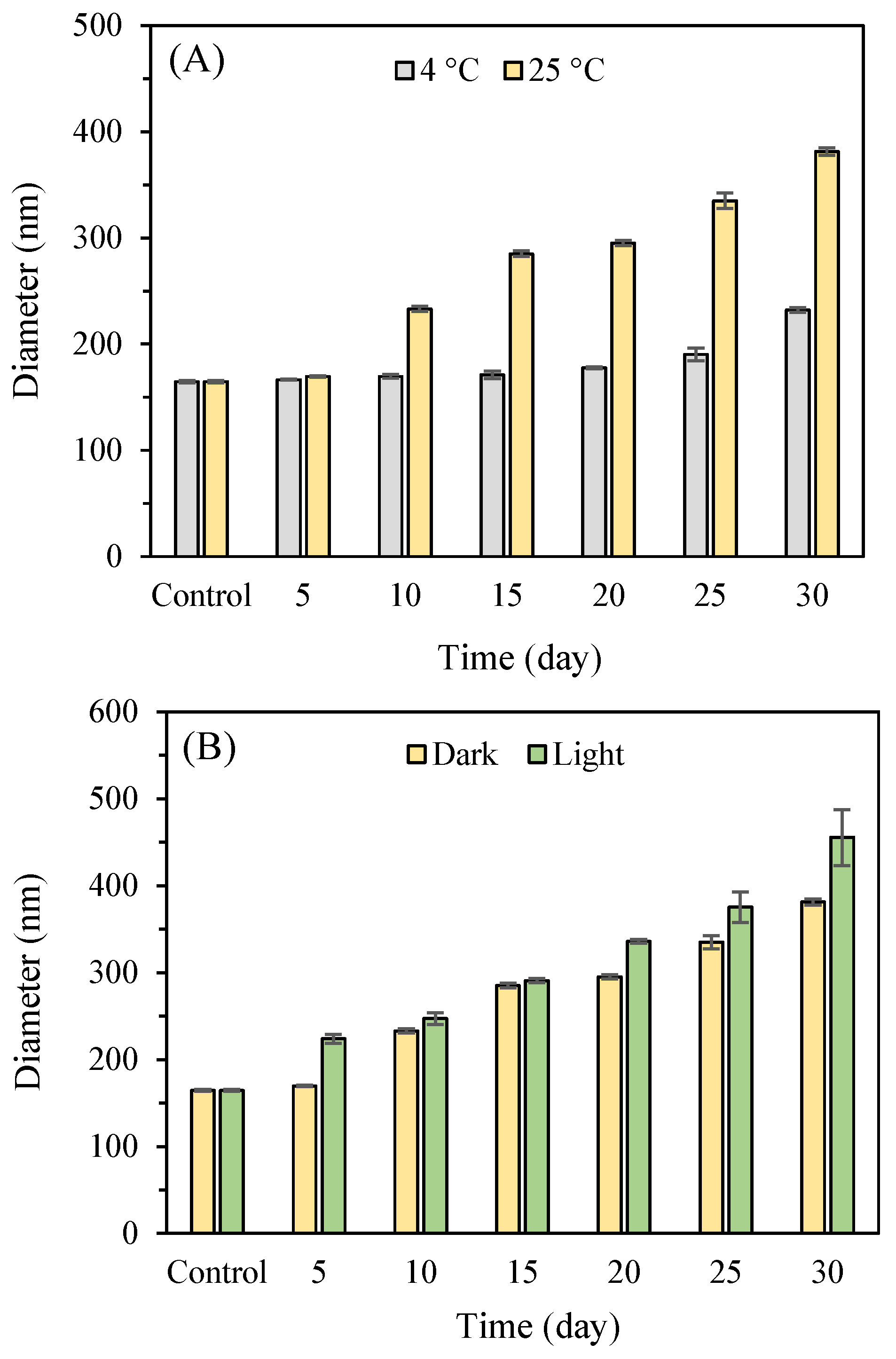
3.4.1. Storage Stability
3.4.2. Influence of pH and Ionic Strength
3.5. Antioxidant Activities of Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. In Nano-Enabled Medical Applications; Jenny Stanford Publishing: Singapore, 2020; pp. 61–91. [Google Scholar]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Weeks, M.E. The discovery of the elements. VI. tellurium and selenium. J. Chem. Educ. 1932, 9, 474. [Google Scholar] [CrossRef]
- Karthik, K.K.; Cheriyan, B.V.; Rajeshkumar, S.; Gopalakrishnan, M. A Review on selenium nanoparticles and their biomedical applications. Biomed. Technol. 2024, 6, 61–74. [Google Scholar] [CrossRef]
- Ye, M.-J.; Xu, Q.-L.; Tang, H.-Y.; Jiang, W.-Y.; Su, D.-X.; He, S.; Zeng, Q.-Z.; Yuan, Y. Development and stability of novel selenium colloidal particles complex with peanut meal peptides. Lwt 2020, 126, 109280. [Google Scholar] [CrossRef]
- Saurav, K.; Mylenko, M.; Ranglová, K.; Kuta, J.; Ewe, D.; Masojídek, J.; Hrouzek, P. In vitro bioaccessibility of selenoamino acids from selenium (Se)-enriched Chlorella vulgaris biomass in comparison to selenized yeast; a Se-enriched food supplement; and Se-rich foods. Food Chem. 2019, 279, 12–19. [Google Scholar]
- Li AiChen, L.A.; Fang Lei, F.L. Optimization of selected parameters affecting selenium content in extracts from Agrocybe cylindracea and anti-fatigue activity of extracts. Acta Edulis Fungi 2015, 22, 75–80. [Google Scholar]
- Huang, J.; Huang, W.; Zhang, Z.; Lin, X.; Lin, H.; Peng, L.; Chen, T. Highly uniform synthesis of selenium nanoparticles with EGFR Targeting and tumor microenvironment-responsive ability for simultaneous diagnosis and therapy of nasopharyngeal carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 11177–11193. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, L.; Feng, L.; Wang, S.; Su, H.; Liu, H.; Liu, H.; Wu, Y. Mitochondrion-targeted selenium nanoparticles enhance reactive oxygen species-mediated cell death. Nanoscale 2020, 12, 1389–1396. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wang, W.-H.; Yang, Y.; Zhang, H.-N. Fabrication and stabilization of biocompatible selenium nanoparticles by Carboxylic curdlans with various molecular properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Zhou, Y.-N.; Li, X.-Y.; Huang, J.; Wahid, F.; Zhong, C.; Chu, L.-Q. Continuous production of antibacterial carboxymethyl chitosan-zinc supramolecular hydrogel fiber using a double-syringe injection device. Int. J. Biol. Macromol. 2020, 156, 252–261. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, T.; Li, J.; Mai, F.; Li, J.; Chen, Y.; Jing, Y.; Dong, X.; Lin, L.; He, J. Selenium nanoparticles as new strategy to potentiate Γδ T Cell anti-tumor cytotoxicity through upregulation of tubulin-α acetylation. Biomaterials 2019, 222, 119397. [Google Scholar] [CrossRef]
- Liu, H.; Lin, W.; He, L.; Chen, T. Radiosensitive core/satellite ternary heteronanostructure for multimodal imaging-guided synergistic cancer radiotherapy. Biomaterials 2020, 226, 119545. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.-Y.; Hong, S.-C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, J.; Wang, H.-Y.; Chen, H.-Y. Synthesis of Selenium nanoparticles in the presence of polysaccharides. Mater. Lett. 2004, 58, 2590–2594. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4. [Google Scholar] [CrossRef]
- Yousaf, M.; Al-Rehaily, A.J.; Ahmad, M.S.; Mustafa, J.; Al-Yahya, M.A.; Al-Said, M.S.; Zhao, J.; Khan, I.A. A 5-alkylresorcinol and three3,4-dihydroisocoumarins derived from Ononis natrix. Phytochem. Lett. 2015, 13, 1–5. [Google Scholar] [CrossRef]
- Bhiri, N.; Hajji, M.; Nasri, R.; Mekki, T.; Nasri, M.; Li, S. Effects of extraction methods on the physicochemical, structural, functional properties and biological activities of extracted polysaccharides from Ononis natrix leaves. Waste Biomass Valorization 2024, 15, 5415–5429. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. i: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (Ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fang, K.; Yuan, H.; Li, D.; Li, H.; Chen, Y.; Luo, X.; Zhang, L.; Ye, X. Acid-induced Poria cocos alkali-soluble polysaccharide hydrogel: Gelation behaviour, characteristics, and potential application in drug delivery. Int. J. Biol. Macromol. 2023, 242, 124383. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef]
- Nan, Z.; Chen, L.; Li, G.; Li, H.; Li, Y.; Ma, J.; Ding, J.; Yang, J. A Method for the quantitative analysis of Lycium barbarum polysaccharides (LBPs) using Fourier-Transform Infrared Spectroscopy (FTIR): From theoretical computation to experimental application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 326, 125204. [Google Scholar] [CrossRef]
- Chen, G.; Fang, C.; Ran, C.; Tan, Y.; Yu, Q.; Kan, J. Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2019, 130, 903–914. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Wang, Z.; Song, W.; Song, H.; Huang, W.; Li, Y.; Feng, J. Effects of extraction methods on the physicochemical properties and functionalities of pectic polysaccharides from burdock (Arctium lappa L.). Int. J. Biol. Macromol. 2024, 257, 128684. [Google Scholar] [CrossRef]
- Xia, F.; Cao, S.; Wang, M.; Sun, Y. Optimizing extraction, structural characterization, and in vitro hypoglycemic activity of a novel polysaccharide component from Lentinus edodes. Food Biosci. 2023, 55, 103007. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and structural characterization of mucilaginous polysaccharides obtained from the seeds of Cassia uniflora for industrial application. Food Chem. 2021, 351, 129262. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural Characterization and antioxidant activity of water-soluble polysaccharides from the tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Wang, X.; An, L.; Bao, J.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.-Q.; Tuerhong, M.; et al. Isolation, Structural elucidation, and immunoregulation properties of an arabinofuranan from the rinds of Garcinia mangostana. Carbohydr. Polym. 2020, 246, 116567. [Google Scholar] [CrossRef] [PubMed]
- Bhotmange, D.U.; Wallenius, J.H.; Singhal, R.S.; Shamekh, S.S. Enzymatic extraction and characterization of polysaccharide from Tuber aestivum. Bioact. Carbohydr. Diet. Fibre 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Sorourian, R.; Khajehrahimi, A.E.; Tadayoni, M.; Azizi, M.H.; Hojjati, M. Ultrasound-Assisted extraction of polysaccharides from Typha domingensis: Structural characterization and functional properties. Int. J. Biol. Macromol. 2020, 160, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, T.; Jin, Z.-Y.; Xu, X.-M.; Wang, J.-H.; Zha, X.-Q.; Chen, H.-Q. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015, 169, 430–438. [Google Scholar] [CrossRef]
- Ding, Z.; Zhao, M.; Li, X.; Wang, X.; Zhang, Z. A Novel polysaccharide from the fruits of Cudrania tricuspidata and its antioxidant and alcohol dehydrogenase activating ability. J. Funct. Foods 2023, 110, 105850. [Google Scholar] [CrossRef]
- Hu, H.; Liang, H.; Wu, Y. Isolation, purification and structural characterization of polysaccharide from Acanthopanax brachypus. Carbohydr. Polym. 2015, 127, 94–100. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Faustino Alves, M.G.D.C.; Pofírio Will, L.S.E.; Costa, T.G.; Sabry, D.A.; De Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A Sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Zou, Y.-F.; Fu, Y.-P.; Chen, X.-F.; Austarheim, I.; Inngjerdingen, K.; Huang, C.; Eticha, L.; Song, X.; Li, L.; Feng, B.; et al. Purification and partial structural characterization of a complement fixating polysaccharide from rhizomes of Ligusticum chuanxiong. Molecules 2017, 22, 287. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Khalifa, R.E.; El Sohaimy, S.A. Functional properties of chia seed mucilage supplemented in low fat yoghurt. Alex. Sci. Exch. J. 2018, 39, 450–459. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Huang, J.-Y.; Ahmad, A. Linum usitatissimum L. seeds: Flax gum extraction, physicochemical and functional characterization. Carbohydr. Polym. 2019, 215, 29–38. [Google Scholar] [CrossRef]
- Ye, F.; Chen, Y.; Liu, J.; Gong, Z.; Zhang, S.; Lin, Q.; Zhou, B.; Liang, Y. A Water-Soluble mycelium polysaccharide from Monascus pilosus: Extraction, structural characterization, immunomodulatory effect and yield enhanced by overexpression of uge gene. Int. J. Biol. Macromol. 2024, 280, 136138. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, X.; Mu, J.; Ho, C.-T.; Su, J.; Zhang, Y.; Lin, X.; Chen, Z.; Li, B.; Xie, Y. Preparation, physicochemical characterization, and anti-proliferation of selenium nanoparticles stabilized by Polyporus umbellatus polysaccharide. Int. J. Biol. Macromol. 2020, 152, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhang, Q.; Guo, J.; Guo, R.; Fan, X.; Bi, Y. Synthesis and evaluation of Grateloupia livida polysaccharides-functionalized selenium nanoparticles. Int. J. Biol. Macromol. 2021, 191, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wu, Y.; Zhang, F.; Zheng, S.; Wang, L.; Bai, J.; Yang, Y. Preparation of Ribes nigrum L. polysaccharides-stabilized selenium nanoparticles for enhancement of the anti-glycation and α-glucosidase inhibitory activities. Int. J. Biol. Macromol. 2023, 253, 127122. [Google Scholar] [CrossRef]
- Wagoner, T.B.; Foegeding, E.A. Whey protein–pectin soluble complexes for beverage applications. Food Hydrocoll. 2017, 63, 130–138. [Google Scholar] [CrossRef]
- Zeng, D.; Zhao, J.; Luk, K.-H.; Cheung, S.-T.; Wong, K.-H.; Chen, T. Potentiation of in vivo anticancer efficacy of selenium nanoparticles by mushroom polysaccharides surface decoration. J. Agric. Food Chem. 2019, 67, 2865–2876. [Google Scholar] [CrossRef]
- Jha, N.; Esakkiraj, P.; Annamalai, A.; Lakra, A.K.; Naik, S.; Arul, V. Synthesis, Optimization, and physicochemical characterization of selenium nanoparticles from polysaccharide of Mangrove rhizophora Mucronata with Potential bioactivities. J. Trace Elem. Miner. 2022, 2, 100019. [Google Scholar] [CrossRef]
- Lin, X.; Mu, J.; Chen, Z.; Zhang, Y.; Ye, X.; Gao, X.; Chen, W.; Luo, Y.; Li, B. Stabilization and functionalization of selenium nanoparticles mediated by green tea and pu-erh tea polysaccharides. Ind. Crops Prod. 2023, 194, 116312. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Qiu, W.-Y.; Sun, L.; Ding, Z.-C.; Yan, J.-K. Preparation, Characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide–protein complexes from Corbicula fluminea. Food Biosci. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Tang, L.; Luo, X.; Wang, M.; Wang, Z.; Guo, J.; Kong, F.; Bi, Y. Synthesis, characterization, in vitro antioxidant and hypoglycemic activities of selenium nanoparticles decorated with polysaccharides of Gracilaria lemaneiformis. Int. J. Biol. Macromol. 2021, 193, 923–932. [Google Scholar] [CrossRef]
- Cao, X.; Xiong, C.; Zhao, X.; Yang, S.; Wen, Q.; Tang, H.; Zeng, Q.; Feng, Y.; Li, J. Tuning self-assembly of amphiphilic sodium alginate-decorated selenium nanoparticle surfactants for antioxidant pickering emulsion. Int. J. Biol. Macromol. 2022, 210, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, R.; Zhou, F.; Wu, Y.; Li, S.; Huo, G.; Ye, J.; Hua, C.; Wang, Z. Preparation, physicochemical characterization, and cytotoxicity of selenium nanoparticles stabilized by Oudemansiella raphanipies polysaccharide. Int. J. Biol. Macromol. 2022, 211, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Caseinate-zein-polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: Effect of polysaccharide type and chemical cross-linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Z.; Hou, J.; Liu, W.; Xu, J.; Guo, Y. Purification and characterization of a chicory polysaccharide and its application in stabilizing genistein for cancer therapy. Int. J. Biol. Macromol. 2023, 242, 124635. [Google Scholar] [CrossRef]
- Cai, W.; Hu, T.; Bakry, A.M.; Zheng, Z.; Xiao, Y.; Huang, Q. Effect of ultrasound on size, morphology, stability and antioxidant activity of selenium nanoparticles dispersed by a hyperbranched polysaccharide from Lignosus rhinocerotis. Ultrason. Sonochemistry 2018, 42, 823–831. [Google Scholar] [CrossRef]
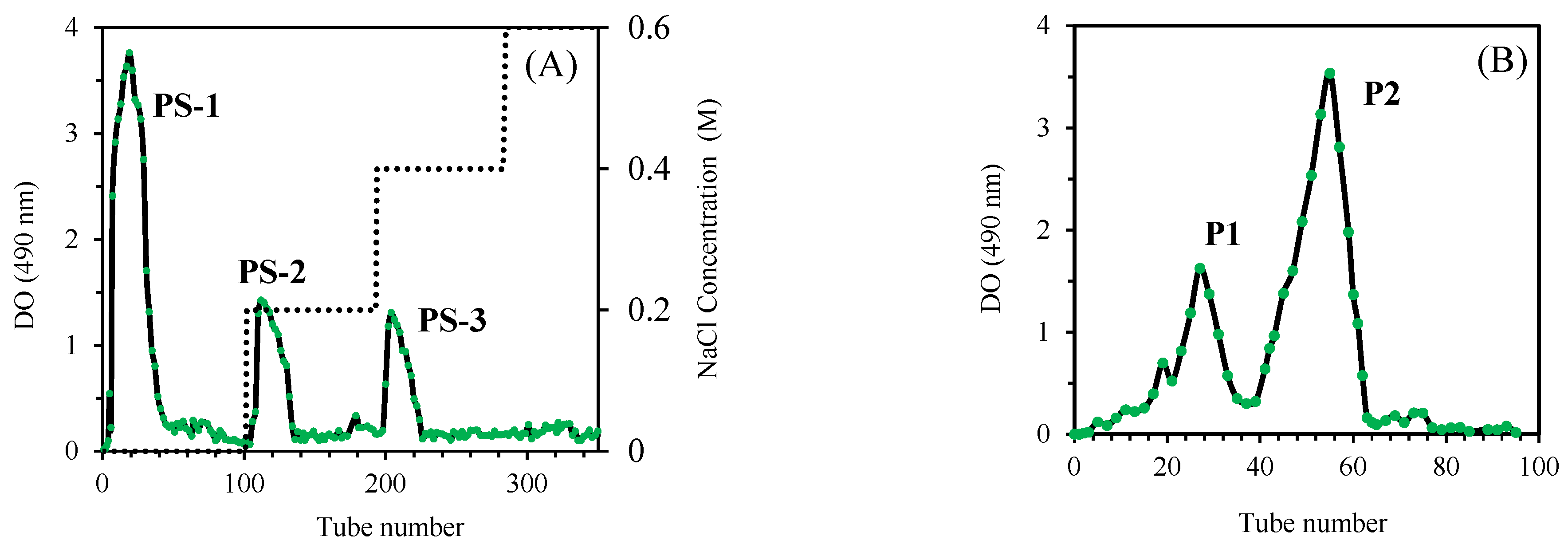



| Mw (kDa) | Ð | |
|---|---|---|
| P1 | ||
| Peak 1 | 732.6 | 1.07 |
| Peak 2 | 74.4 | 1.6 |
| P2 | 30.2 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhiri, N.; Masquelez, N.; Nasri, M.; Nasri, R.; Hajji, M.; Li, S. Synthesis, Characterization, and Stability Study of Selenium Nanoparticles Coated with Purified Polysaccharides from Ononis natrix. Nanomaterials 2025, 15, 435. https://doi.org/10.3390/nano15060435
Bhiri N, Masquelez N, Nasri M, Nasri R, Hajji M, Li S. Synthesis, Characterization, and Stability Study of Selenium Nanoparticles Coated with Purified Polysaccharides from Ononis natrix. Nanomaterials. 2025; 15(6):435. https://doi.org/10.3390/nano15060435
Chicago/Turabian StyleBhiri, Nour, Nathalie Masquelez, Moncef Nasri, Rim Nasri, Mohamed Hajji, and Suming Li. 2025. "Synthesis, Characterization, and Stability Study of Selenium Nanoparticles Coated with Purified Polysaccharides from Ononis natrix" Nanomaterials 15, no. 6: 435. https://doi.org/10.3390/nano15060435
APA StyleBhiri, N., Masquelez, N., Nasri, M., Nasri, R., Hajji, M., & Li, S. (2025). Synthesis, Characterization, and Stability Study of Selenium Nanoparticles Coated with Purified Polysaccharides from Ononis natrix. Nanomaterials, 15(6), 435. https://doi.org/10.3390/nano15060435







