Graphene-Supported Cun (n = 5, 6) Clusters for CO2 Reduction Catalysis
Abstract
:1. Introduction
2. Calculation Method
3. Results and Discussion
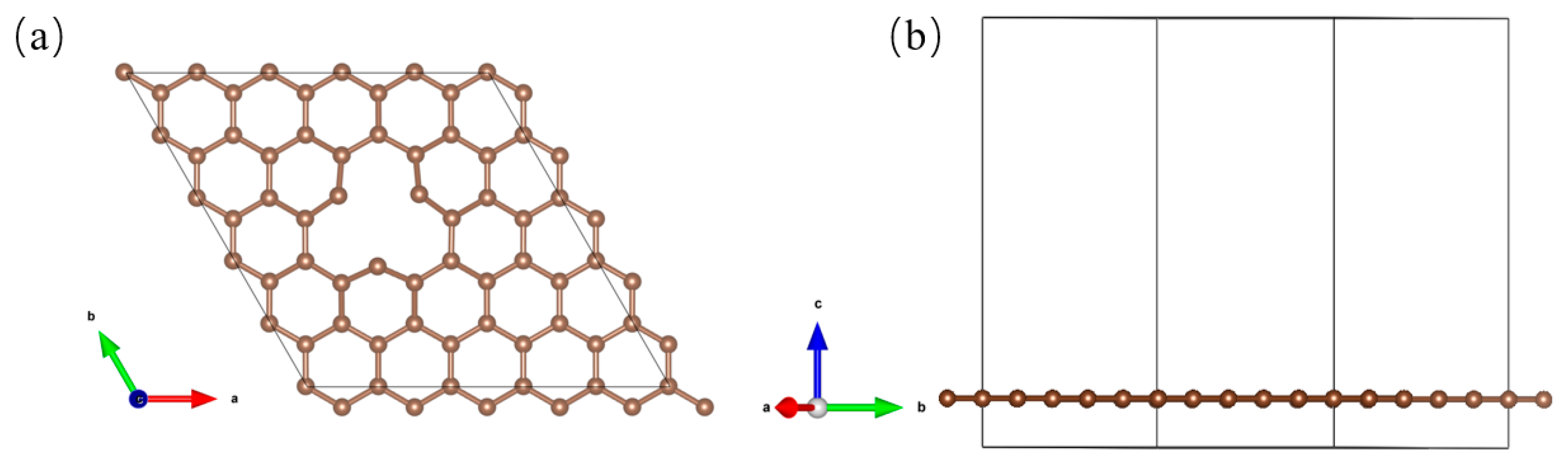
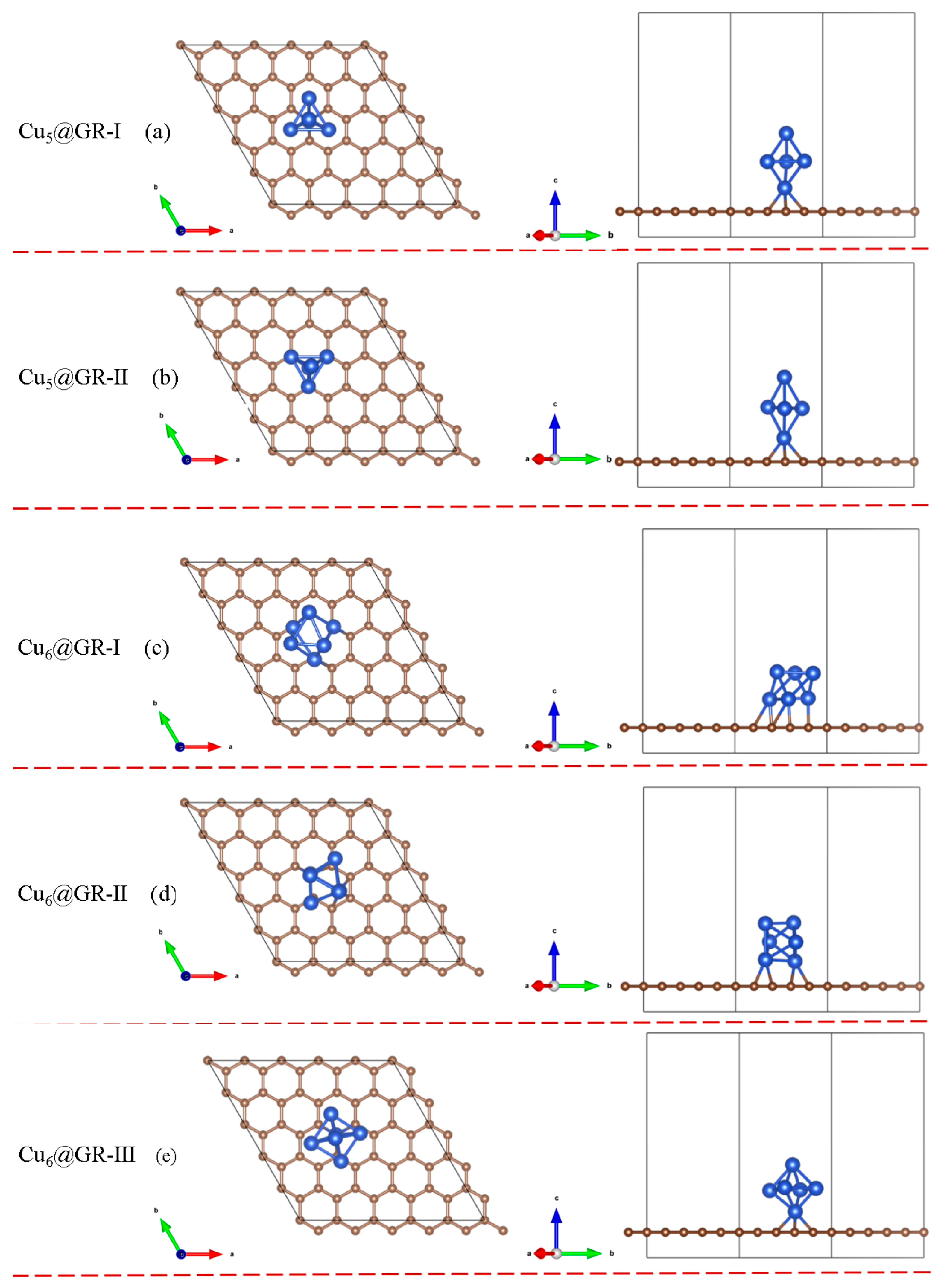
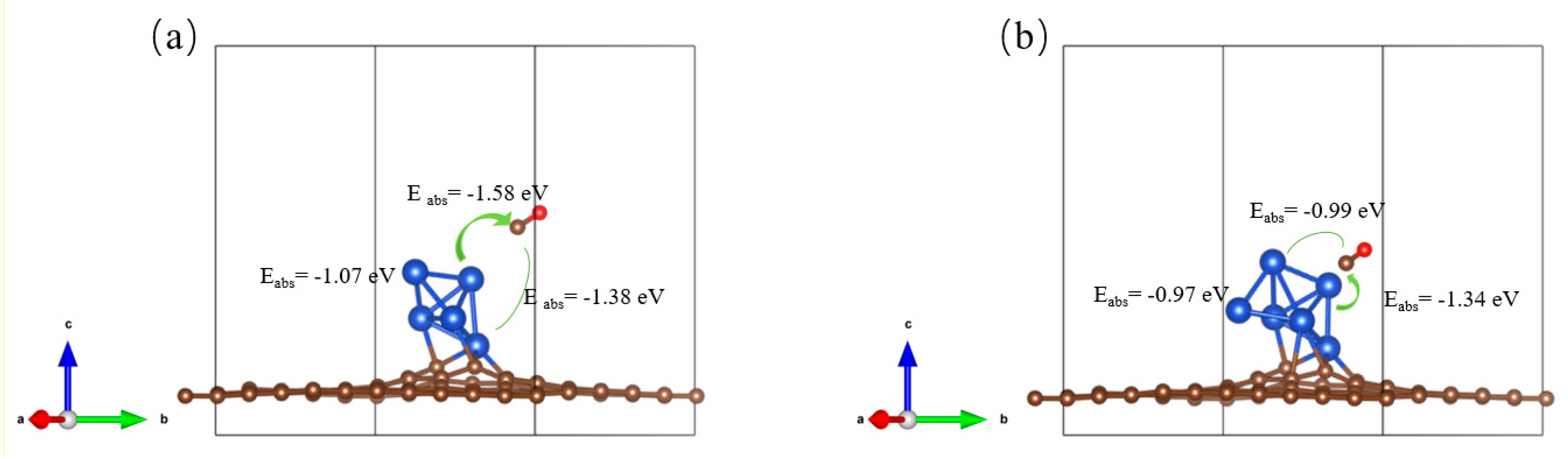
3.1. Catalyst Structure
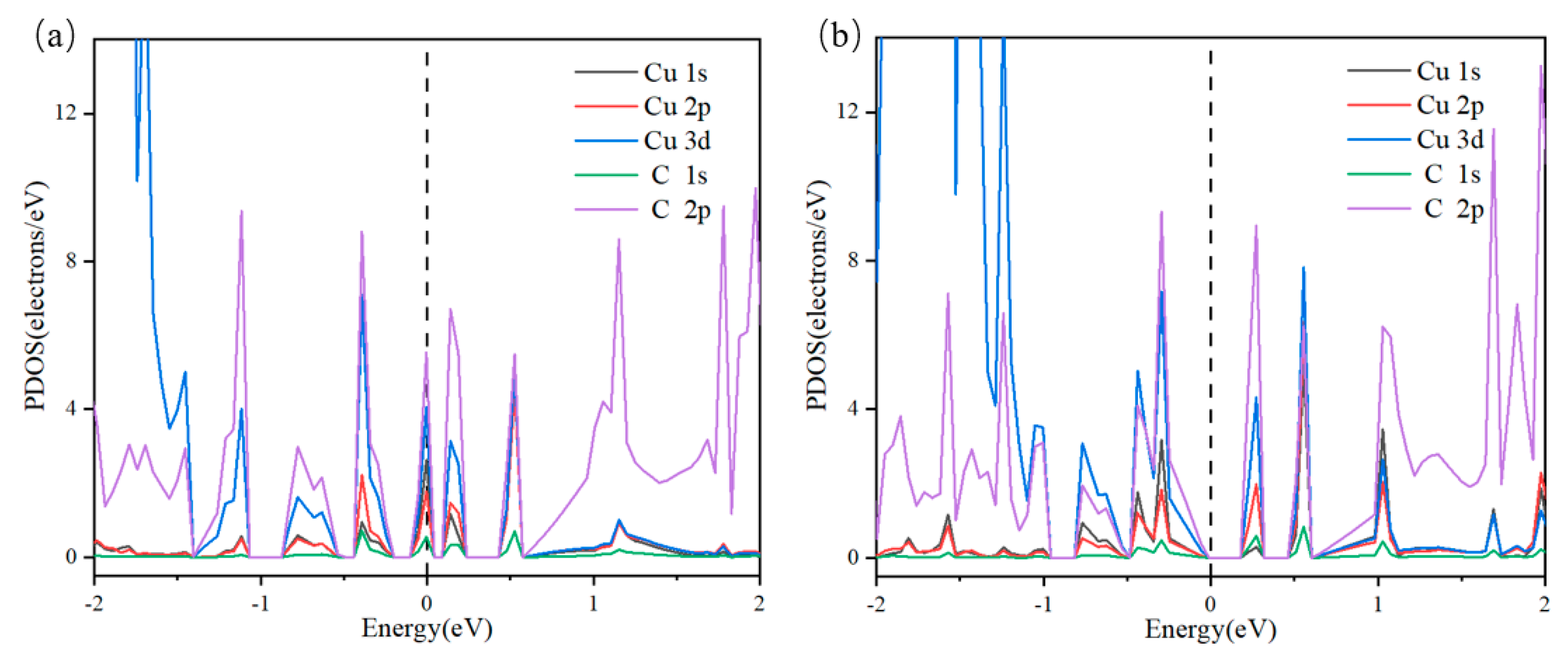
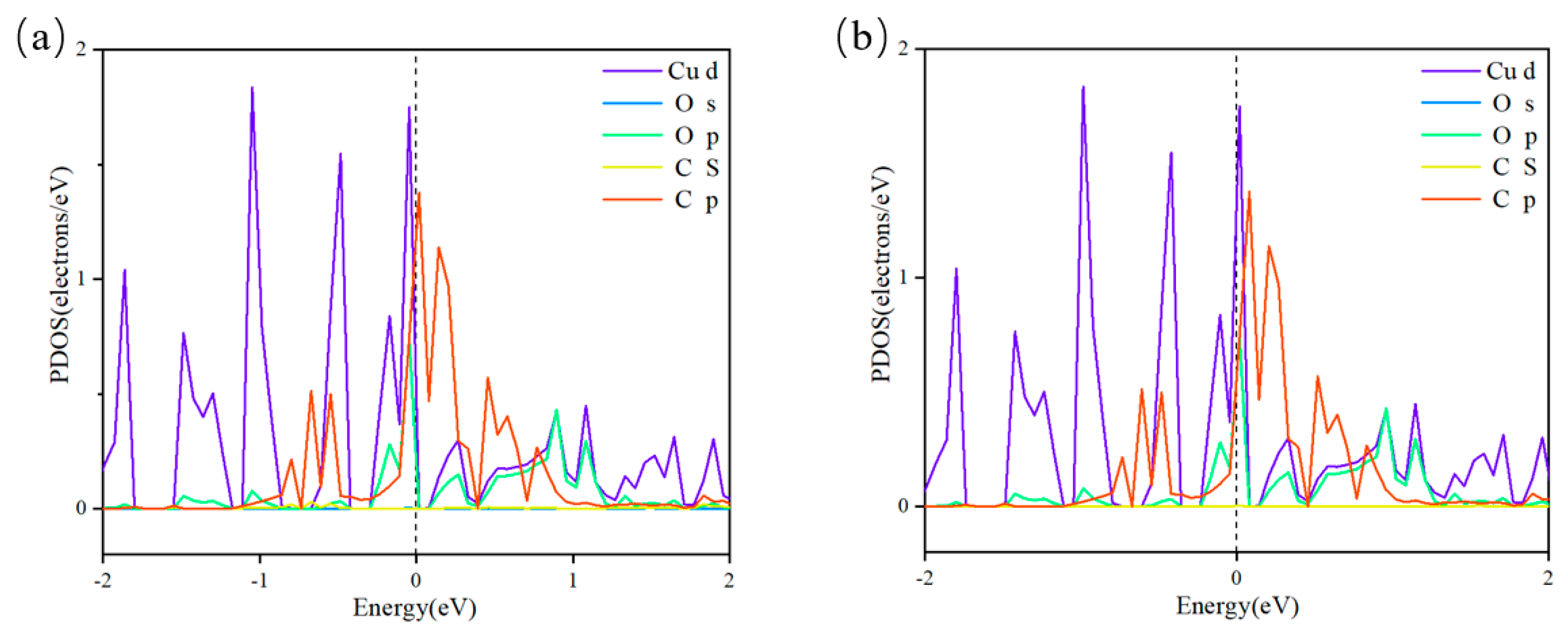
3.2. Catalytic Activity
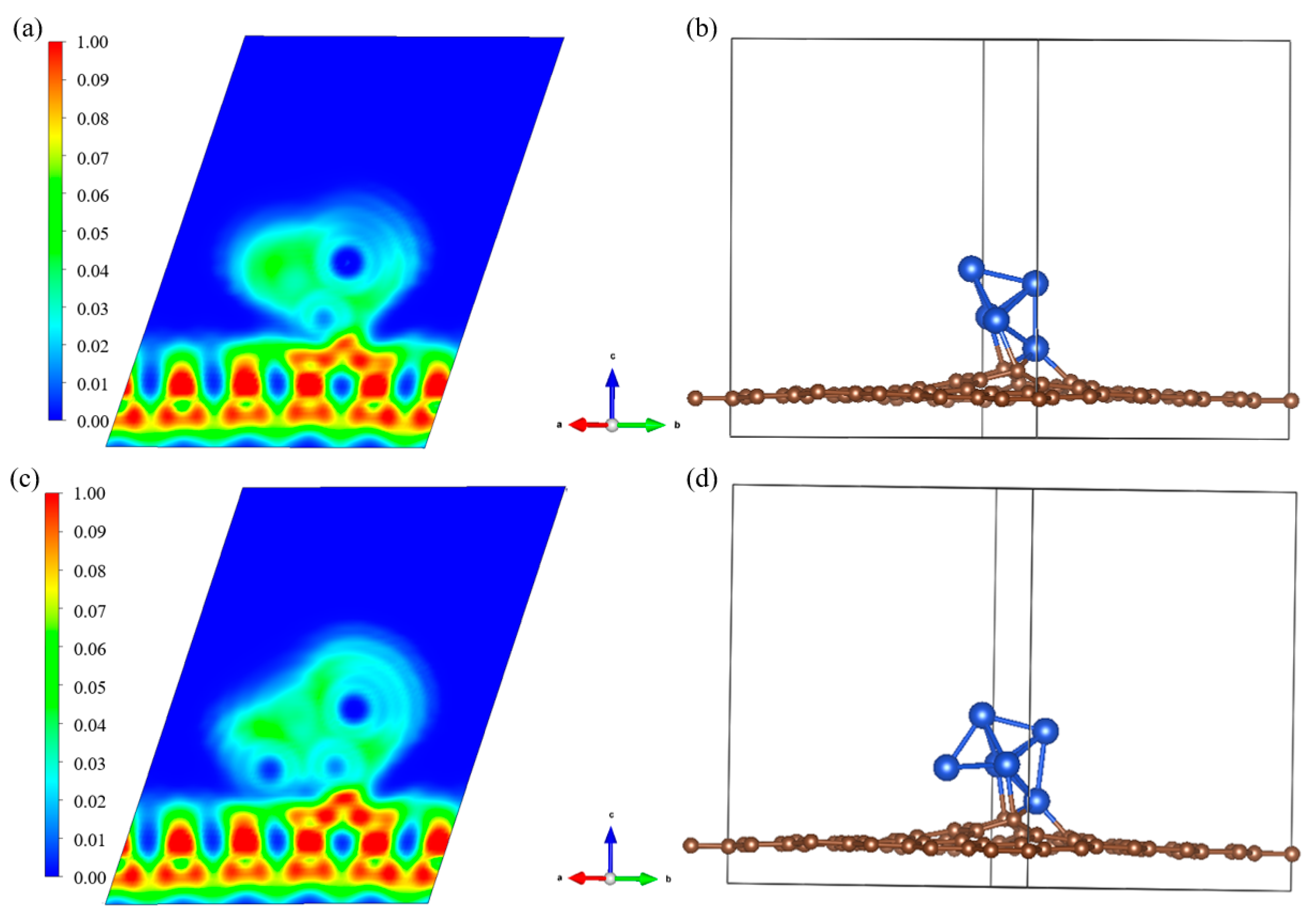
3.3. Reaction Site
3.4. Catalytic Process
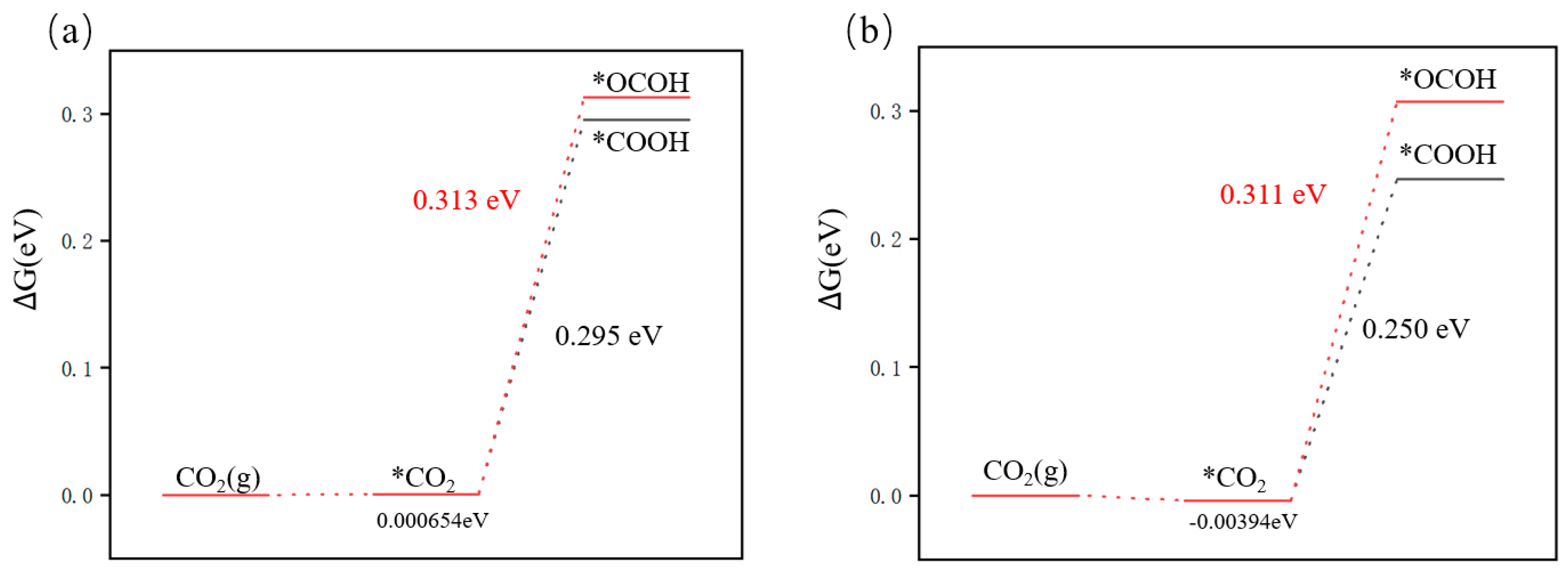
3.4.1. First Step of Protonation
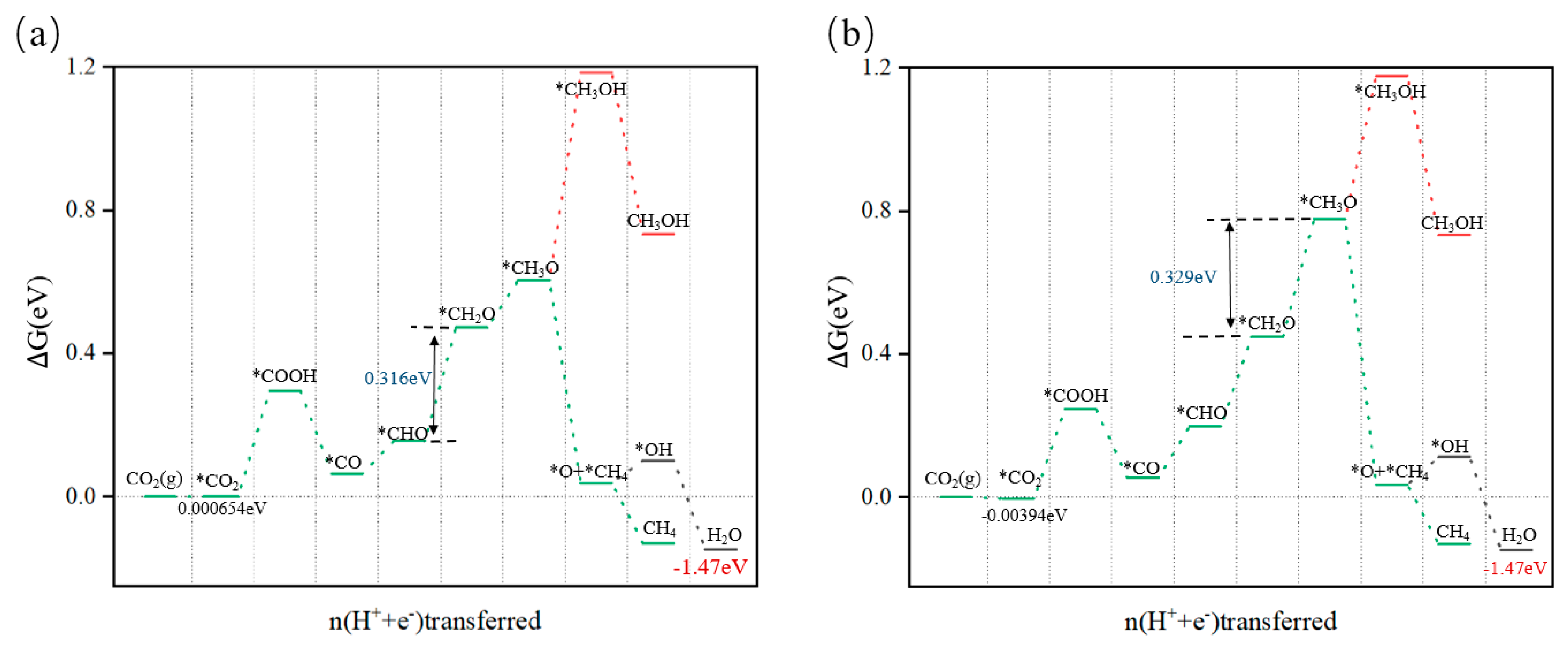
3.4.2. Reaction Pathway
3.5. Analysis of Side Reactions
Hydrogen Evolution Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Zhang, J.; Hua, M.; Wan, Q.; Su, Z.; Tan, X.; Liu, L.; Zhang, F.; Chen, G.; Tan, D.; et al. Highly Electrocatalytic Ethylene Production from CO2 on Nanodefective Cu Nanosheets. J. Am. Chem. Soc. 2020, 142, 13606–13613. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Kwon, S.; Cheng, T.; Xu, M.; Tieu, P.; Lee, C.; Cai, J.; Lee, H.M.; Pan, X.; Duan, X.; et al. Highly Active and Stable Stepped Cu Surface for Enhanced Electrochemical CO2 Reduction to C2H4. Nat. Catal. 2020, 3, 804–812. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, O.; Lv, S.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef]
- Jin, L.; Liu, B.; Wang, P.; Yao, H.; Achola, L.A.; Kerns, P.; Lopes, A.; Yang, Y.; Ho, J.; Moewes, A.; et al. Ultrasmall Au Nanocatalysts Supported on Nitrided Carbon for Electrocatalytic CO2 Reduction: The Role of the Carbon Support in High Selectivity. Nanoscale 2018, 10, 14678–14686. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wu, Y.; Luo, Y.; Zhou, N. Double atom-anchored Defective Boron Nitride catalyst for efficient electroreduction of CO2 to CH4: A first principles study. Chem. Phys. Lett. 2020, 756, 137852. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Hydrogen Carbonate Solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Cheng, T.; Xiao, H.; Goddard, W.A., III. Reaction Mechanisms for the Electrochemical Reduction of CO2 to CO and Formate on the Cu (100) Surface at 298 K from Quantum Mechanics Free Energy Calculations with Explicit Water. J. Am. Chem. Soc. 2016, 138, 13802–13805. [Google Scholar] [CrossRef]
- Du, D.; Zhu, H.; Guo, Y.; Hong, X.; Zhang, Q.; Suo, B.; Zou, W.; Li, Y. Anchoring Cu Clusters Over Defective Graphene for Electrocatalytic Reduction of CO2. J. Phys. Chem. C 2022, 126, 11611–11618. [Google Scholar] [CrossRef]
- Pan, F.; Fang, L.; Li, B.; Yang, X.; O’Carroll, T.; Li, H.; Li, T.; Wang, G.; Chen, K.-J.; Wu, G. N and OH-Immobilized Cu3 Clusters In Situ Reconstructed from Single-Metal Sites for Efficient CO2 Electromethanation in Bicontinuous Mesochannels. J. Am. Chem. Soc. 2024, 146, 1423–1434. [Google Scholar] [CrossRef]
- Haunschild, R.; Barth, A.; Marx, W. Evolution of DFT Studies in View of a Scientometric Perspective. J. Chem. Inf. Model. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J. Ab Initio Simulations of Materials Using VASP: Density-Functional Theory and Beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Chu, W.; Zheng, Q.; Akimov, A.V.; Zhao, J.; Saidi, W.A.; Prezhdo, O.V. Accurate Computation of Nonadiabatic Coupling with Projector Augmented-Wave Pseudopotentials. J. Phys. Chem. Lett. 2020, 11, 10073–10080. [Google Scholar] [CrossRef]
- Pašti, I.A.; Jovanović, A.; Dobrota, A.S.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Atomic Adsorption on Pristine Graphene Along the Periodic Table of Elements–From PBE to Non-local Functionals. Appl. Surf. Sci. 2018, 436, 433–440. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Durgun, E.; Bilc, D.I.; Ciraci, S.; Ghosez, P. Hydrogen-Saturated Silicon Nanowires Heavily Doped with Interstitial and Substitutional Transition Metals. J. Phys. Chem. C 2012, 116, 15713–15722. [Google Scholar] [CrossRef]
- Li, Y.; Su, H.; Chan, S.H.; Sun, Q. CO2 Electroreduction Performance of Transition Metal Dimers Supported on Graphene: A Theoretical Study. ACS Catal. 2015, 5, 6658–6664. [Google Scholar] [CrossRef]
- Huang, F.; Deng, Y.; Chen, Y.; Cai, X.; Peng, M.; Jia, Z.; Xie, J.; Xiao, D.; Wen, X.; Wang, N.; et al. Anchoring Cu1 Species Over Nanodiamond-Graphene for Semi-Hydrogenation of Acetylene. Nat. Commun. 2019, 10, 4431. [Google Scholar] [CrossRef]
- Kyriakou, V.; Vourros, A.; Garagounis, I.; Carabineiro, S.A.C.; Maldonado-Hódar, F.J.; Marnellos, G.E.; Konsolakis, M. Highly Active and Stable TiO2-Supported Au Nanoparticles for CO2 Reduction. Catal. Commun. 2017, 98, 52–56. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Y.; Zhu, H.; Su, H.; Chan, S.H.; Sun, Q. Enhanced CO2 Electroreduction on Armchair Graphene Nanoribbons Edge-Decorated with Copper. Nano Res. 2017, 10, 1641–1650. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of Hydrocarbons in the Electrochemical Reduction of Carbon Dioxide at a Copper Electrode in Aqueous Solution. J. Chem. Soc. Faraday Trans. 1 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Addison, D. A Viewpoint on Heterogeneous Electrocatalysis and Redox Mediation in Nonaqueous Li-O2 Batteries. ACS Catal. 2017, 7, 772–778. [Google Scholar] [CrossRef]


| CO2 | CO2 in Cu5@GR | CO2 in Cu6@GR | |
|---|---|---|---|
| C | 1.889397 | 1.885506 | 1.895131 |
| O-1 | 7.051664 | 7.077166 | 7.070804 |
| O-2 | 7.058943 | 7.072862 | 7.087998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, L.; Zou, Y.; Wang, X.; Ning, Q. Graphene-Supported Cun (n = 5, 6) Clusters for CO2 Reduction Catalysis. Nanomaterials 2025, 15, 445. https://doi.org/10.3390/nano15060445
Guo Y, Zhang L, Zou Y, Wang X, Ning Q. Graphene-Supported Cun (n = 5, 6) Clusters for CO2 Reduction Catalysis. Nanomaterials. 2025; 15(6):445. https://doi.org/10.3390/nano15060445
Chicago/Turabian StyleGuo, Yanling, Lisu Zhang, Yanbo Zou, Xingguo Wang, and Qian Ning. 2025. "Graphene-Supported Cun (n = 5, 6) Clusters for CO2 Reduction Catalysis" Nanomaterials 15, no. 6: 445. https://doi.org/10.3390/nano15060445
APA StyleGuo, Y., Zhang, L., Zou, Y., Wang, X., & Ning, Q. (2025). Graphene-Supported Cun (n = 5, 6) Clusters for CO2 Reduction Catalysis. Nanomaterials, 15(6), 445. https://doi.org/10.3390/nano15060445







