Ellipsometric Surface Oxidation Model of ALD-Grown Vanadium Oxide Mixed-Valence System
Abstract
1. Introduction
2. Materials and Methods
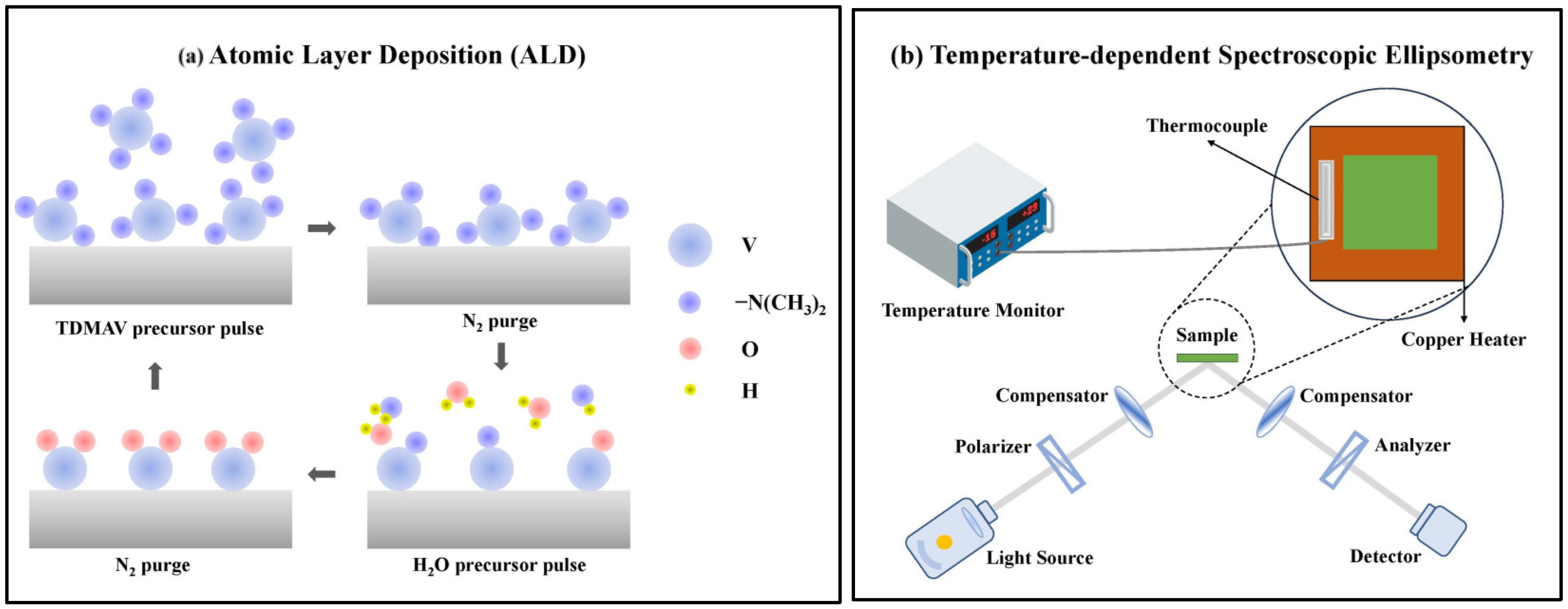
2.1. Thin Film Fabrication
2.2. Thin Film Characterization
2.3. Methods
3. Results and Discussion
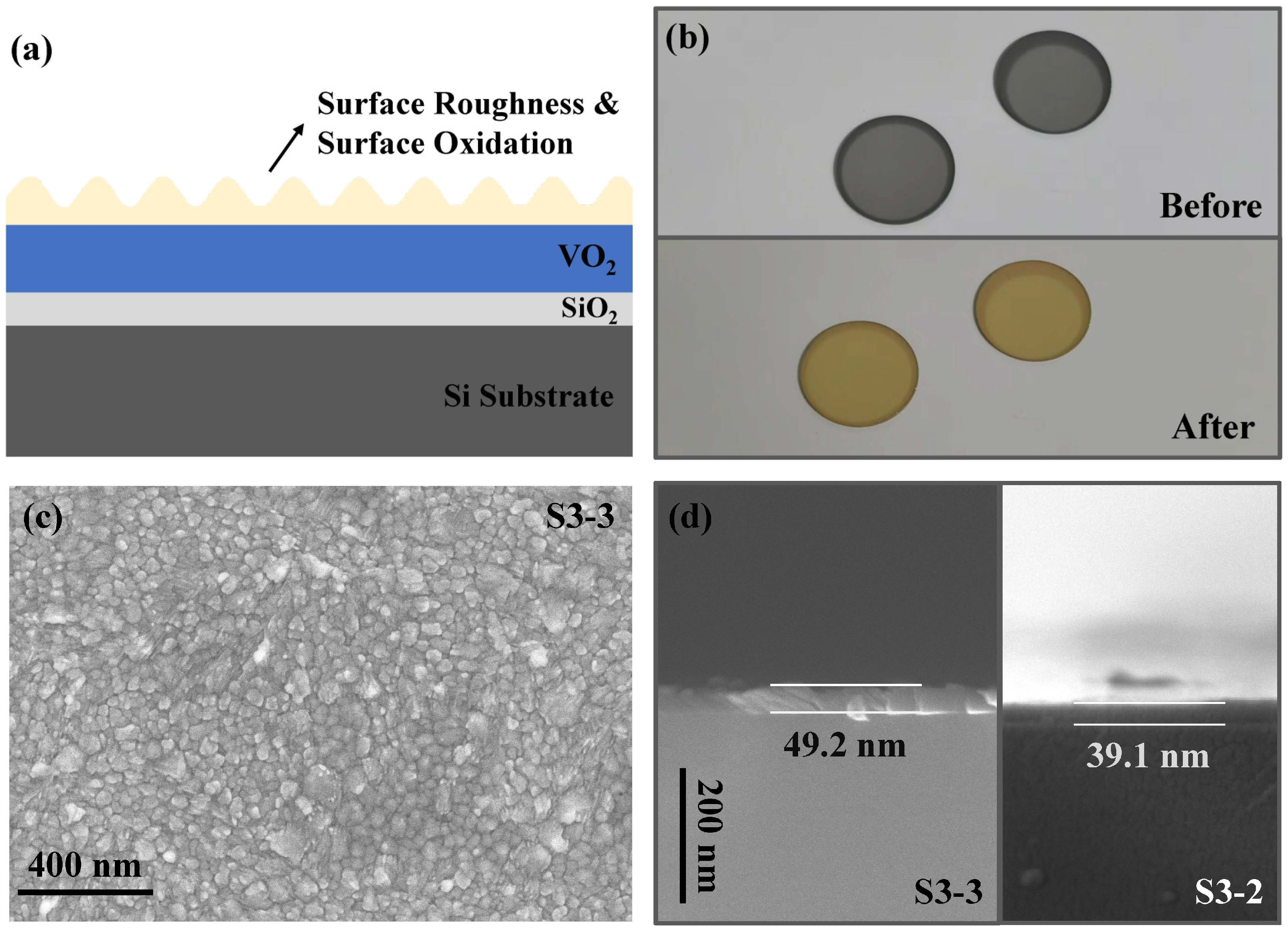
3.1. Film Deposition and Post-Annealing
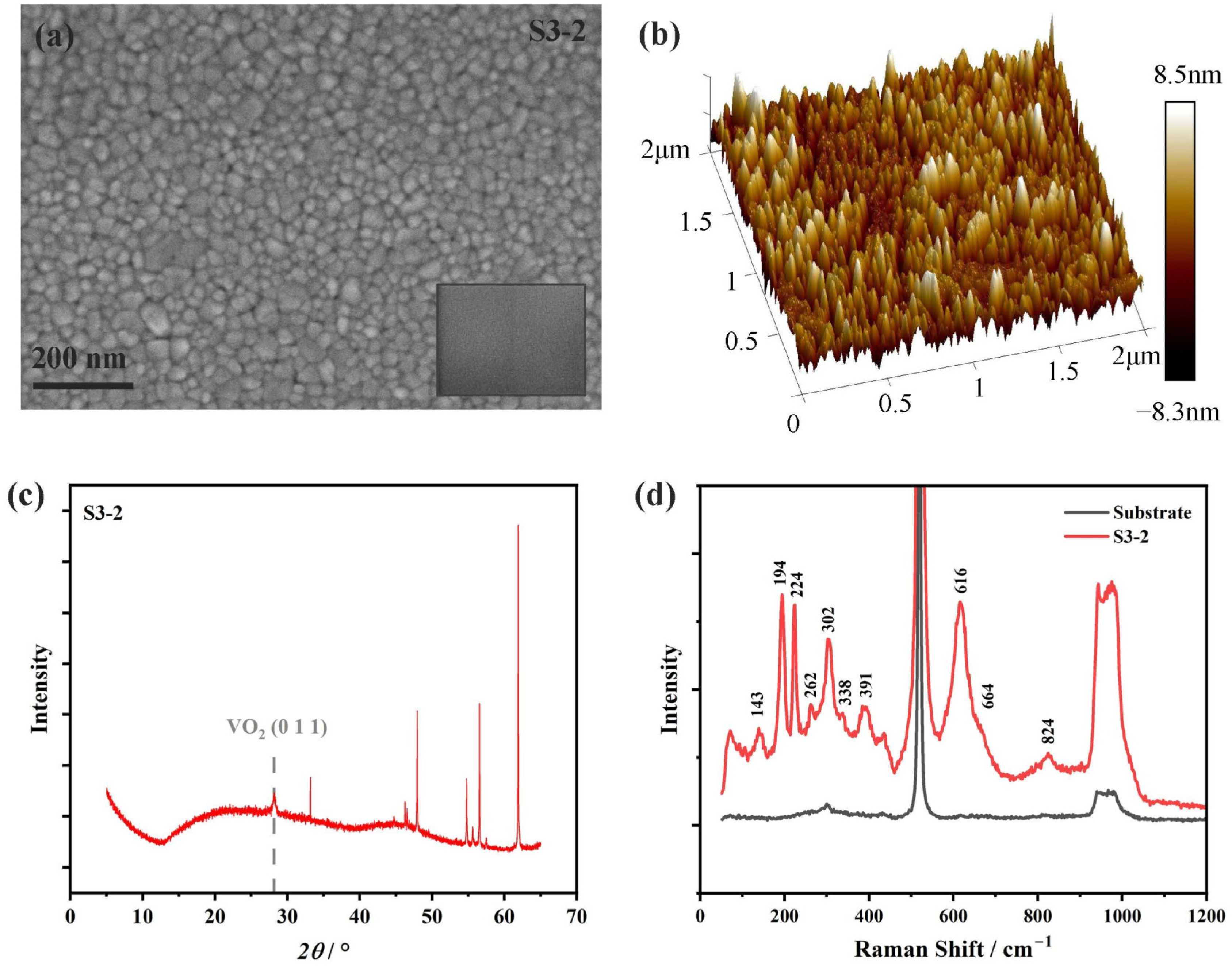
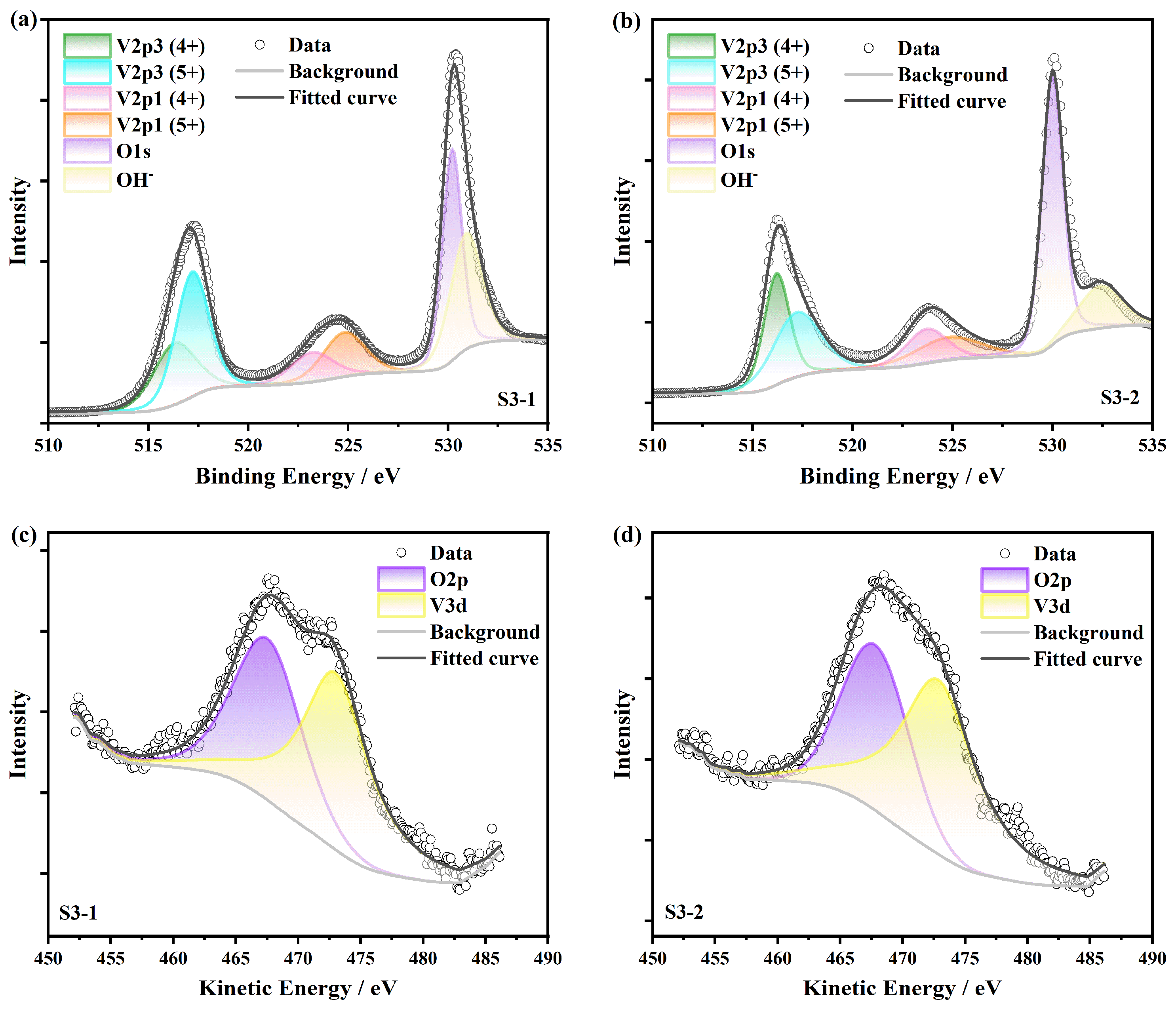
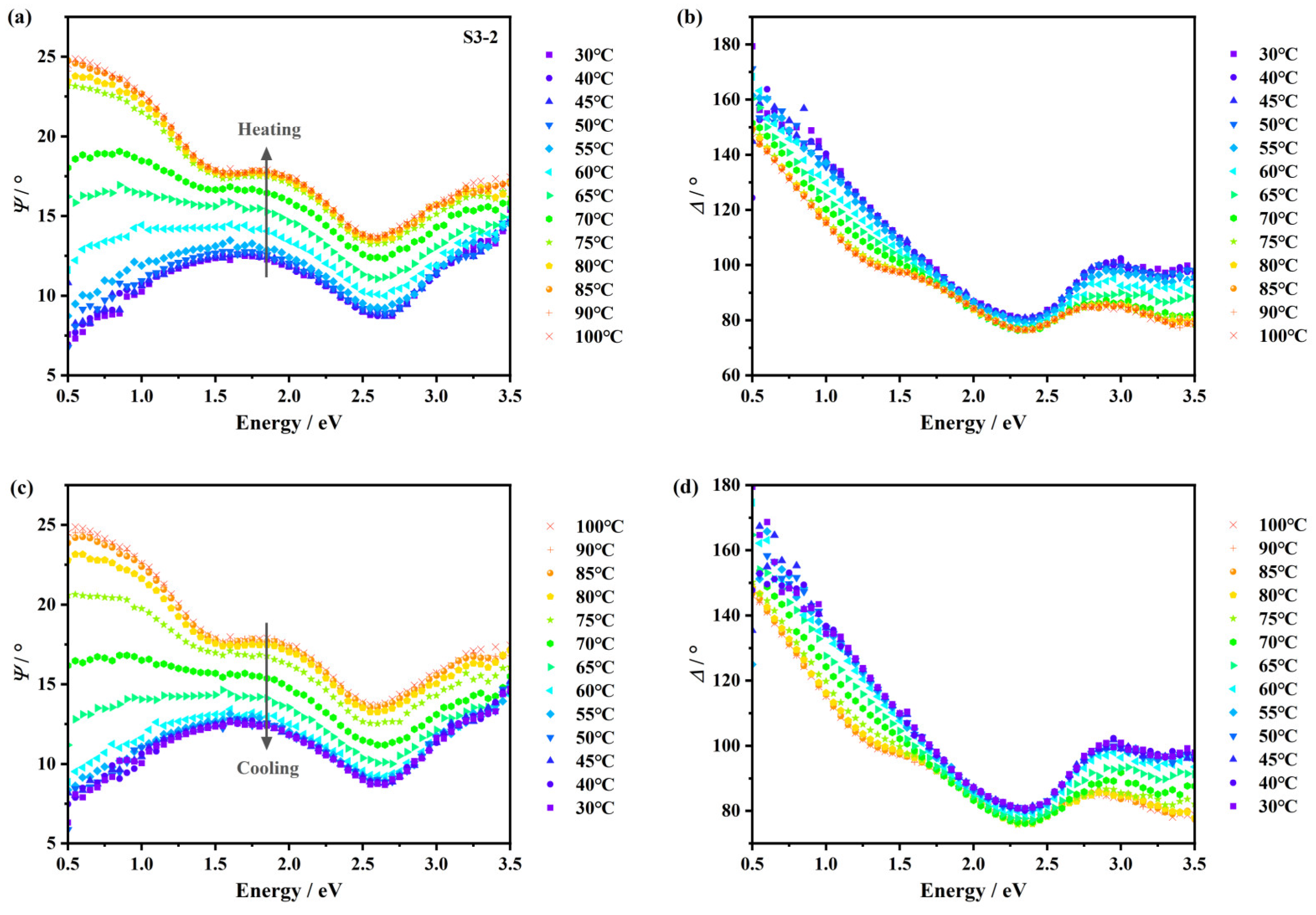
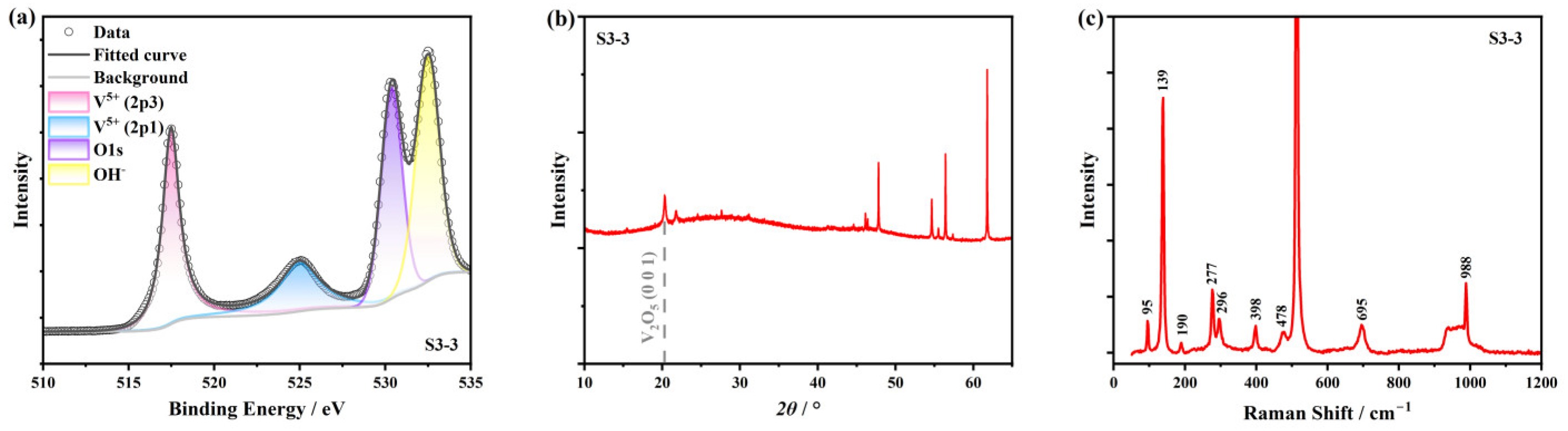
3.2. XPS Characterization and Ellipsometry Modeling
3.3. Surface Oxidation Model and Air Thermal Oxidation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Atomic layer deposition |
| XPS | X-ray photoelectron spectroscopy |
| AES | Auger electron spectroscopy |
| SOM | Surface oxidation model |
| ATO | Air thermal oxidation |
| XRD | X-ray diffraction |
| SE | Spectroscopic ellipsometry |
| GPC | Growth rate per cycle |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
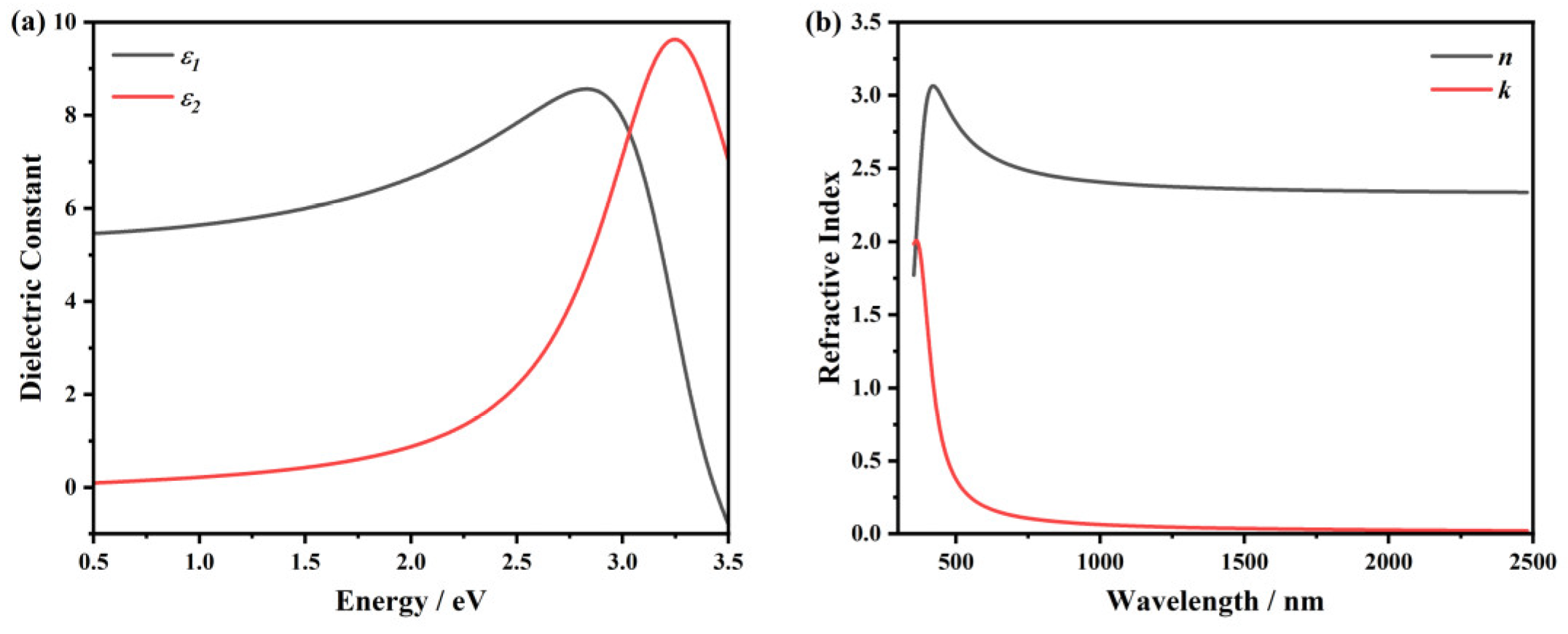
Appendix A. Ellipsometry Models
| S3-1 | 2.75 | 0.47 | / | / | 1.43 | 10.22 | 2.68 | 2.53 | 2.86 | 1.54 | |
| S3-2 | M1 | 3.85 | 0.50 | / | / | 0.54 | 26.15 | 19.12 | 3.20 | 9.09 | 1.00 |
| R | 0.36 | 0 | 4.33 | 0.68 | 3.17 | 8.41 | 1.32 | / | / | / | |
| S3-3 | 2.75 | 0.79 | / | / | 3.23 | 14.35 | 0.98 | / | / | / | |
References
- Shvets, P.; Dikaya, O.; Maksimova, K.; Goikhman, A. A review of Raman spectroscopy of vanadium oxides. J. Raman Spectrosc. 2019, 50, 1226–1244. [Google Scholar] [CrossRef]
- Bahlawane, N.; Lenoble, D. Vanadium Oxide Compounds: Structure, Properties, and Growth from the Gas Phase. Chem. Vap. Depos. 2014, 20, 299–311. [Google Scholar] [CrossRef]
- Shao, Z.; Cao, X.; Luo, H.; Jin, P. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 2018, 10, 581–605. [Google Scholar] [CrossRef]
- Liu, K.; Lee, S.; Yang, S.; Delaire, O.; Wu, J. Recent progresses on physics and applications of vanadium dioxide. Mater. Today 2018, 21, 875–896. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, S.; Liu, G.; Li, M.; White, T.J.; Long, Y. Vanadium Dioxide: The Multistimuli Responsive Material and Its Applications. Small 2018, 14, e1802025. [Google Scholar] [CrossRef]
- Qazilbash, M.M.; Brehm, M.; Chae, B.-G.; Ho, P.-C.; Andreev, G.O.; Kim, B.-J.; Yun, S.J.; Balatsky, A.V.; Maple, M.B.; Keilmann, F.; et al. Mott Transition in VO2 Revealed by Infrared Spectroscopy and Nano-Imaging. Science 2007, 318, 1750–1753. [Google Scholar] [CrossRef]
- Park, J.H.; Coy, J.M.; Kasirga, T.S.; Huang, C.; Fei, Z.; Hunter, S.; Cobden, D.H. Measurement of a solid-state triple point at the metal–insulator transition in VO2. Nature 2013, 500, 431–434. [Google Scholar] [CrossRef]
- Ashok, P.; Chauhan, Y.S.; Verma, A. Vanadium dioxide thin films synthesized using low thermal budget atmospheric oxidation. Thin Solid Films 2020, 706, 138003. [Google Scholar] [CrossRef]
- Pilloud, D.; García-Wong, A.C.; Mangin, D.; Capon, F.; Pierson, J.F. A comparative study of the thermochromic performances of VO2 films obtained by air oxidation of V and VN precursors. Sol. Energy Mater. Sol. Cells 2022, 248, 111947. [Google Scholar] [CrossRef]
- Li, Z.; Cao, C.; Li, M.; Wang, L.; Zhu, D.; Xu, F.; Huang, A.; Jin, P.; Yu, L.; Cao, X. Gradient Variation Oxygen-Content Vanadium–Oxygen Composite Films with Enhanced Crystallinity and Excellent Durability for Smart Windows. ACS Appl. Mater. Interfaces 2023, 15, 9401–9411. [Google Scholar] [CrossRef]
- Matsuda, M.; Nagata, M.; Akamine, H.; Shida, K.; Matsuda, M. Microstructural characterization of oxygen-defective VO2-x films produced by precisely controlled oxidation of vanadium metal foil. J. Ceram. Soc. Jpn. 2025, 133, 9–14. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, Q.; Wang, T.; Wang, S.; Li, N.; Lin, G.; Liu, X.; Dai, Z.; Yan, J.; Yin, J.; et al. Cephalopod-inspired versatile design based on plasmonic VO2 nanoparticle for energy-efficient mechano-thermochromic windows. Nano Energy 2020, 73, 104785. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, T.; Meng, Y.; Yang, R.; Tan, G.; Long, Y. Scalable thermochromic smart windows with passive radiative cooling regulation. Science 2021, 374, 1501–1504. [Google Scholar] [CrossRef]
- Kwon, S.-i.; Hagiwara, M.; Kim, S.-h.; Fujihara, S. Multistep synthesis of VO2 (M) nanoparticles and their application to thermochromic hybrid films for IR modulation. Int. J. Appl. Ceram. Technol. 2023, 20, 1997–2008. [Google Scholar] [CrossRef]
- Hu, X.; Cai, W.; Zhang, Y.; Shi, S.; Ming, Y.; Yu, R.; Chen, D.; Yang, M.; Wang, F.; Yang, H.; et al. Facile and Widely Applicable Route to Self-Adaptive Emissivity Modulation: Energy-Saving Demonstration with Transparent Wood. Nano Lett. 2024, 24, 657–666. [Google Scholar] [CrossRef]
- Bhupathi, S.; Wang, S.; Wang, G.; Long, Y. Porous vanadium dioxide thin film-based Fabry−Perot cavity system for radiative cooling regulating thermochromic windows: Experimental and simulation studies. Nanophotonics 2024, 13, 711–723. [Google Scholar] [CrossRef]
- Ono, M.; Chen, K.; Li, W.; Fan, S. Self-adaptive radiative cooling based on phase change materials. Opt. Express 2018, 26, A777–A787. [Google Scholar] [CrossRef]
- Kort-Kamp, W.J.M.; Kramadhati, S.; Azad, A.K.; Reiten, M.T.; Dalvit, D.A.R. Passive Radiative “Thermostat” Enabled by Phase-Change Photonic Nanostructures. ACS Photonics 2018, 5, 4554–4560. [Google Scholar] [CrossRef]
- Tang, K.; Dong, K.; Li, J.; Gordon, M.P.; Reichertz, F.G.; Kim, H.; Rho, Y.; Wang, Q.; Lin, C.-Y.; Grigoropoulos, C.P.; et al. Temperature-adaptive radiative coating for all-season household thermal regulation. Science 2021, 374, 1504–1509. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; He, R.; Guo, J.; Zhang, R.; Zheng, Y.; Chen, L.-Y. Vanadium dioxide-based metasurface for temperature-adaptive radiative cooling. Opt. Eng. 2025, 64, 027102. [Google Scholar] [CrossRef]
- Ao, X.; Li, B.; Zhao, B.; Hu, M.; Ren, H.; Yang, H.; Liu, J.; Cao, J.; Feng, J.; Yang, Y.; et al. Self-adaptive integration of photothermal and radiative cooling for continuous energy harvesting from the sun and outer space. Proc. Natl. Acad. Sci. USA 2022, 119, e2120557119. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.; Kruzelecky, R.V.; Murzionak, P.; Jamroz, W.; Tagziria, K.; Chaker, M.; Ledrogoff, B. Review of the VO2 smart material applications with emphasis on its use for spacecraft thermal control. Front. Mater. 2022, 9, 1013848. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, F.; Wang, L.; Chen, R.; Wu, J.; Grigoropoulos, C.P.; Yao, J. Phase change materials in photonic devices. J. Appl. Phys. 2021, 129, 030902. [Google Scholar] [CrossRef]
- An, Y.; Fu, Y.; Dai, J.-G.; Yin, X.; Lei, D. Switchable radiative cooling technologies for smart thermal management. Cell Rep. Phys. Sci. 2022, 3, 101098. [Google Scholar] [CrossRef]
- Xu, F.; Cao, X.; Luo, H.; Jin, P. Recent advances in VO2-based thermochromic composites for smart windows. J. Mater. Chem. C 2018, 6, 1903–1919. [Google Scholar] [CrossRef]
- Cui, Y.; Ke, Y.; Liu, C.; Chen, Z.; Wang, N.; Zhang, L.; Zhou, Y.; Wang, S.; Gao, Y.; Long, Y. Thermochromic VO2 for Energy-Efficient Smart Windows. Joule 2018, 2, 1707–1746. [Google Scholar] [CrossRef]
- Hryha, E.; Rutqvist, E.; Nyborg, L. Stoichiometric vanadium oxides studied by XPS. Surf. Interface Anal. 2012, 44, 1022–1025. [Google Scholar] [CrossRef]
- Ureña-Begara, F.; Crunteanu, A.; Raskin, J.-P. Raman and XPS characterization of vanadium oxide thin films with temperature. Appl. Surf. Sci. 2017, 403, 717–727. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Film. 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Prasadam, V.P.; Bahlawane, N.; Mattelaer, F.; Rampelberg, G.; Detavernier, C.; Fang, L.; Jiang, Y.; Martens, K.; Parkin, I.P.; Papakonstantinou, I. Atomic layer deposition of vanadium oxides: Process and application review. Mater. Today Chem. 2019, 12, 396–423. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, Y.; Yan, L.; Li, H.; Su, Y.; Meng, H.; Wang, X. Efficient Charge Injection in Organic Field-Effect Transistors Enabled by Low-Temperature Atomic Layer Deposition of Ultrathin VOx Interlayer. Adv. Funct. Mater. 2016, 26, 4456–4463. [Google Scholar] [CrossRef]
- Lv, X.; Cao, Y.; Yan, L.; Li, Y.; Song, L. Atomic layer deposition of VO2 films with Tetrakis-dimethyl-amino vanadium (IV) as vanadium precursor. Appl. Surf. Sci. 2017, 396, 214–220. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Gao, Y.; Wang, J. Atomic layer deposition of vanadium oxide thin films from tetrakis(dimethylamino)vanadium precursor. J. Mater. Res. 2017, 32, 37–44. [Google Scholar] [CrossRef]
- Lv, X.; Cao, Y.; Yan, L.; Li, Y.; Zhang, Y.; Song, L. Atomic Layer Deposition of V1–xMoxO2 Thin Films, Largely Enhanced Luminous Transmittance, Solar Modulation. ACS Appl. Mater. Interfaces 2018, 10, 6601–6607. [Google Scholar] [CrossRef]
- Schilbe, P. Raman scattering in VO2. Phys. B Condens. Matter 2002, 316–317, 600–602. [Google Scholar] [CrossRef]
- Su, Q.; Liu, X.Q.; Ma, H.L.; Guo, Y.P.; Wang, Y.Y. Raman spectroscopic characterization of the microstructure of V2O5 films. J. Solid State Electrochem. 2008, 12, 919–923. [Google Scholar] [CrossRef]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-valence vanadium oxides studied by XPS. Surf. Sci. 2000, 454–456, 41–44. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Lindberg, B.J.; Hamrin, K.; Johansson, G.; Gelius, U.; Fahlman, A.; Nordling, C.; Siegbahn, K. Molecular Spectroscopy by Means of ESCA II. Sulfur compounds. Correlation of electron binding energy with structure. Phys. Scr. 1970, 1, 286. [Google Scholar] [CrossRef]
- Sawatzky, G.A.; Post, D. X-ray photoelectron and Auger spectroscopy study of some vanadium oxides. Phys. Rev. B 1979, 20, 1546–1555. [Google Scholar] [CrossRef]
- Hilfiker, J.; Tompkins, H. Spectroscopic Ellipsometry: Practical Application to Thin Film Characterization; Momentum Press: New York, NY, USA, 2016. [Google Scholar]
- Fujiwara, H. Principles of Spectroscopic Ellipsometry. In Spectroscopic Ellipsometry; Wiley: Hoboken, NJ, USA, 2007; pp. 81–146. [Google Scholar]
- Chen, X.; Liu, S. Optical Scatterometry for Nanostructure Metrology. In Metrology; Gao, W., Ed.; Springer: Singapore, 2019; pp. 1–37. [Google Scholar]
- Orji, N.G.; Badaroglu, M.; Barnes, B.M.; Beitia, C.; Bunday, B.D.; Celano, U.; Kline, R.J.; Neisser, M.; Obeng, Y.; Vladar, A.E. Metrology for the next generation of semiconductor devices. Nat. Electron. 2018, 1, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-T.; He, R.; Peng, L.; Yang, Y.-T.; Sun, X.-J.; Zhang, Y.-S.; Zheng, Y.-X.; Liu, B.-J.; Zhang, R.-J.; Wang, S.-Y.; et al. Interpretation of Reflection and Colorimetry Characteristics of Indium-Particle Films by Means of Ellipsometric Modeling. Nanomaterials 2023, 13, 383. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, Z.; Woolf, D.; Hessel, C.M.; Rensberg, J.; Hensley, J.M.; Xiao, Y.; Shahsafi, A.; Salman, J.; Richter, S.; et al. On the Optical Properties of Thin-Film Vanadium Dioxide from the Visible to the Far Infrared. Ann. Physik 2019, 531, 1900188. [Google Scholar] [CrossRef]
- Nazari, M.; Zhao, Y.; Kuryatkov, V.V.; Fan, Z.Y.; Bernussi, A.A.; Holtz, M. Temperature dependence of the optical properties of VO2 deposited on sapphire with different orientations. Phys. Rev. B 2013, 87, 035142. [Google Scholar] [CrossRef]
- Kana Kana, J.B.; Vignaud, G.; Gibaud, A.; Maaza, M. Thermally driven sign switch of static dielectric constant of VO2 thin film. Opt. Mater. 2016, 54, 165–169. [Google Scholar] [CrossRef]
- Abdelkadir, A.A.; Victor, J.-L.; Vignaud, G.; Marcel, C.; Sahal, M.; Maaza, M.; Chaker, M.; Gibaud, A. Analysis of the temperature dependent optical properties of V1−xWxO2 thin films. Thin Solid Films 2023, 772, 139805. [Google Scholar] [CrossRef]
- Oukassi, S.; Salot, R.; Pereira-Ramos, J.P. Elaboration and characterization of crystalline RF-deposited V2O5 positive electrode for thin film batteries. Appl. Surf. Sci. 2009, 256, 149–155. [Google Scholar] [CrossRef]
- Losurdo, M.; Barreca, D.; Bruno, G.; Tondello, E. Spectroscopic ellipsometry investigation of V2O5 nanocrystalline thin films. Thin Solid Films 2001, 384, 58–64. [Google Scholar] [CrossRef]
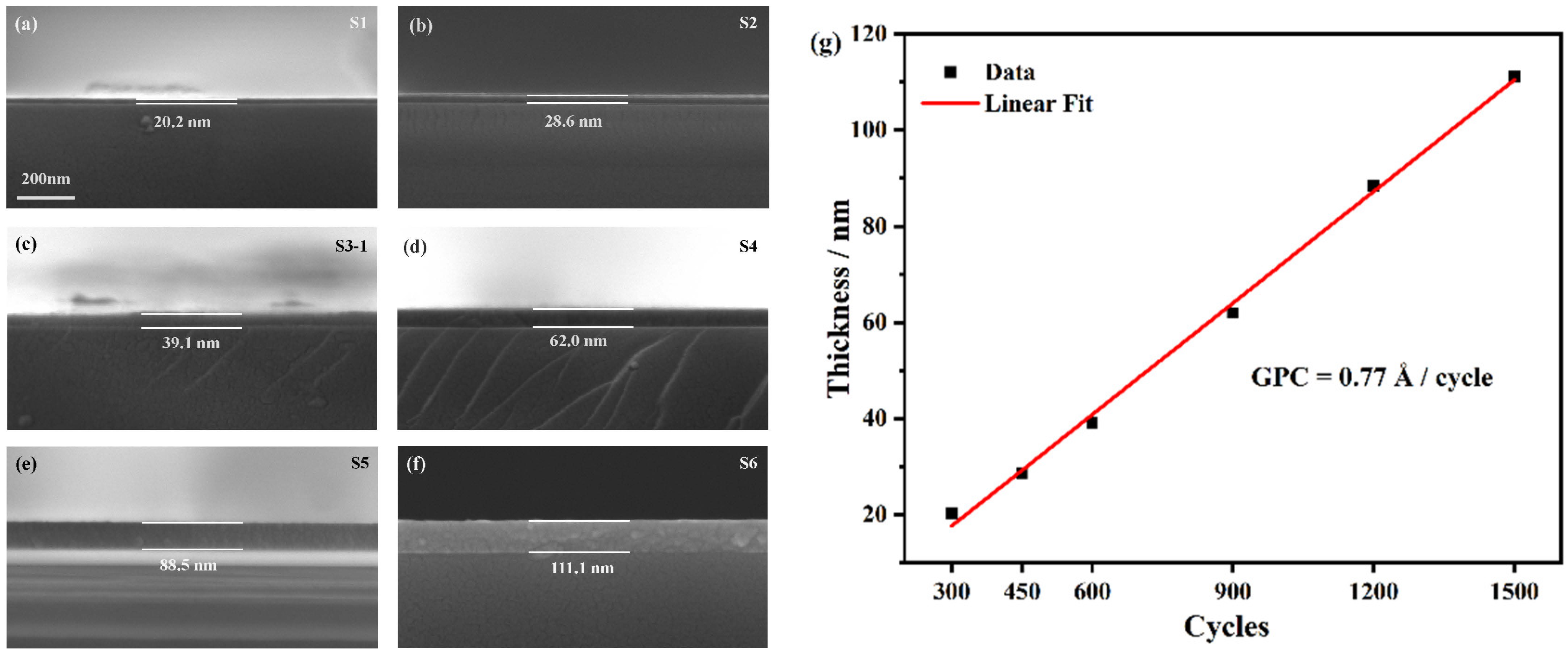

| ALD Cycles | 300 Cycles | 450 Cycles | 600 Cycles | 900 Cycles | 1200 Cycles | 1500 Cycles |
|---|---|---|---|---|---|---|
| Sample No. | S1 | S2 | S3-1 (“Amo”) | S4 | S5 | S6 |
| S3-2 (“Ann”) | ||||||
| S3-3 (“ATO”) |
| VO2 Ratio | V2O5 Ratio | O2p: V3d | x (VOx) | Mixed-Valence | |
|---|---|---|---|---|---|
| S3-1 | 38.64% | 61.36% | 0.81 | 2.31 | 4.62 |
| S3-2 | 48.24% | 51.76% | 0.71 | 2.26 | 4.52 |
| Thickness/nm | SO | SR | VO2 | SiO2 |
|---|---|---|---|---|
| S3-1 | 6.0 | 0 | 33.7 | 1.7 |
| S3-2 | 3.1 | 1.8 | 36.3 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, S.; Cai, Q.; Liu, J.; Li, C.; Hu, E.; Li, J.; Wang, S.; Zheng, Y.; Chen, L.; et al. Ellipsometric Surface Oxidation Model of ALD-Grown Vanadium Oxide Mixed-Valence System. Nanomaterials 2025, 15, 645. https://doi.org/10.3390/nano15090645
Sun X, Wang S, Cai Q, Liu J, Li C, Hu E, Li J, Wang S, Zheng Y, Chen L, et al. Ellipsometric Surface Oxidation Model of ALD-Grown Vanadium Oxide Mixed-Valence System. Nanomaterials. 2025; 15(9):645. https://doi.org/10.3390/nano15090645
Chicago/Turabian StyleSun, Xiaojie, Shuguang Wang, Qingyuan Cai, Jingze Liu, Changhai Li, Ertao Hu, Jing Li, Songyou Wang, Yuxiang Zheng, Liangyao Chen, and et al. 2025. "Ellipsometric Surface Oxidation Model of ALD-Grown Vanadium Oxide Mixed-Valence System" Nanomaterials 15, no. 9: 645. https://doi.org/10.3390/nano15090645
APA StyleSun, X., Wang, S., Cai, Q., Liu, J., Li, C., Hu, E., Li, J., Wang, S., Zheng, Y., Chen, L., & Lee, Y. (2025). Ellipsometric Surface Oxidation Model of ALD-Grown Vanadium Oxide Mixed-Valence System. Nanomaterials, 15(9), 645. https://doi.org/10.3390/nano15090645








