Scindapsus Aureus Resistive Random-Access Memory with Synaptic Plasticity and Sound Localization Function
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pang, J.; Peng, S.; Hou, C.; Wang, X.; Wang, T.; Cao, Y.; Zhou, W.; Sun, D.; Wang, K.; Rümmeli, M.; et al. Applications of MXenes in human-like sensors and actuators. Nano Res. 2023, 16, 5767–5795. [Google Scholar]
- Di Liberto, G.M.; O’sullivan, J.A.; Lalor, E.C. Low-frequency cortical entrainment to speech reflects phoneme-level processing. Curr. Biol. 2015, 25, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Kell, A.J.E.; Yamins, D.L.K.; Shook, E.N.; Norman-Haignere, S.V.; McDermott, J.H. A task-optimized neural network replicates human auditory behavior, predicts brain responses, and reveals a cortical processing hierarchy. Neuron 2018, 98, 630–644.e16. [Google Scholar] [CrossRef] [PubMed]
- Tichacek, O.; Mistrík, P.; Jungwirth, P. From the outer ear to the nerve: A complete computer model of the peripheral auditory system. Hear. Res. 2023, 440, 108900. [Google Scholar] [CrossRef]
- Yun, S.Y.; Han, J.K.; Lee, S.W.; Yu, J.M.; Jeon, S.B.; Choi, Y.K. Self-aware artificial auditory neuron with a triboelectric sensor for spike-based neuromorphic hardware. Nano Energy 2023, 109, 108322. [Google Scholar] [CrossRef]
- Liu, Y.; Li, E.; Wang, X.; Chen, Q.; Zhou, Y.; Hu, Y.; Chen, G.; Chen, H.; Guo, T. Self-powered artificial auditory pathway for intelligent neuromorphic computing and sound detection. Nano Energy 2020, 78, 105403. [Google Scholar] [CrossRef]
- Escudero, E.C.; Peña, F.P.; Vicente, R.P.; Jimenez-Fernandez, A.; Moreno, G.J.; Morgado-Estevez, A. Real-time neuro-inspired sound source localization and tracking architecture applied to a robotic platform. Neurocomputing 2018, 283, 129–139. [Google Scholar] [CrossRef]
- Grothe, B.; Pecka, M.; McAlpine, D. Mechanisms of sound localization in mammals. Physiol. Rev. 2010, 90, 983–1012. [Google Scholar] [CrossRef]
- Wang, Y.; Mandal, S. Bioinspired radio-frequency source localization based on cochlear cross-correlograms. Front. Neurosci. 2021, 15, 623316. [Google Scholar]
- Wang, Y.; Mendis, G.J.; Wei-Kocsis, J.; Madanayake, A.; Mandal, S. A 1.0–8.3 GHz cochlea-based real-time spectrum analyzer with Δ-Σ-modulated digital outputs. IEEE Trans. Circuits Syst. I Regul. Pap. 2020, 67, 2934–2947. [Google Scholar] [CrossRef]
- Xu, Y.; Afshar, S.; Wang, R.; Thakur, C.S.; van Schaik, A. A biologically inspired sound localization system using a silicon cochlea pair. Appl. Sci. 2021, 11, 1519. [Google Scholar] [CrossRef]
- Nguyen, D.; Aarabi, P.; Sheikholeslami, A. Real-time sound localization using field-programmable gate arrays. In Proceedings of the 2003 International Conference on Multimedia and Expo (ICME’03), Baltimore, MD, USA, 6–9 July 2003; IEEE: New York, NY, USA; Volume 2, pp. II-829–II-832. [Google Scholar]
- Jungdong, J. Real-time sound localization using generalized cross correlation based on 0.13 μm CMOS process. J. Semicond. Technol. Sci. 2014, 14, 175–183. [Google Scholar]
- Zhang, W.; Gao, B.; Tang, J.; Yao, P.; Yu, S.; Chang, M.F.; Yoo, H.J.; He, Q.; Wu, H. Neuro-inspired computing chips. Nat. Electron. 2020, 3, 371–382. [Google Scholar] [CrossRef]
- Xi, Y.; Gao, B.; Tang, J.; Chen, A.; Chang, M.F.; Hu, X.S.; Van Der Spiegel, J.; He, Q.; Wu, H. In-Memory Learning with Analog Resistive Switching Memory: A Review and Perspective. Proc. IEEE 2020, 109, 1–29. [Google Scholar] [CrossRef]
- Gao, S.; Liu, G.; Chen, Q.; Xue, W.; Yang, H.; Shang, J.; Chen, B.; Zeng, F.; Song, C.; Pan, F.; et al. Improving unipolar resistive switching uniformity with cone-shaped conducting filaments and its logic-in-memory application. ACS Appl. Mater. Interfaces 2018, 10, 6453–6462. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Su, W.; Wen, D. Nonvolatile Bioresistive Random Access Memory Based on Glycine max and Graphene Oxide. ACS Appl. Electron. Mater. 2023, 5, 5814–5822. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, H.; Zuo, Z.; Wen, D. Full-function logic circuit based on egg albumen resistive memory. Appl. Phys. Lett. 2022, 121, 243505. [Google Scholar] [CrossRef]
- Wang, L.; Wei, S.; Xie, J.; Wen, D. Bioartificial Synapses for Neuromorphic Computing. ACS Sustain. Chem. Eng. 2023, 11, 2229–2237. [Google Scholar] [CrossRef]
- Wang, L.; Xie, J.; Wen, D. Forming-free plant resistive random access memory based on the Coulomb blockade effect produced by gold nanoparticles. Phys. Chem. Chem. Phys. 2023, 25, 18132–18138. [Google Scholar] [CrossRef]
- Kumar, S.; Davila, N.; Wang, Z.; Huang, X.; Strachan, J.P.; Vine, D.; Kilcoyne, D.A.L.; Nishibayashi, Y.; Williams, R.S. Spatially uniform resistance switching of low current, high endurance titanium–niobium-oxide memristors. Nanoscale 2017, 9, 1793–1798. [Google Scholar] [CrossRef]
- Papadopoulos, S.; Agarwal, T.; Jain, A.; Taniguchi, T.; Watanabe, K.; Luisier, M.; Emboras, A.; Novotny, L. Ion Migration in Monolayer Mo S 2 Memristors. Phys. Rev. Appl. 2022, 18, 014018. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Su, W.; Wen, D. Photoelectric biomemristors for artificial visual perception systems. Appl. Mater. Today 2023, 35, 101964. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.; Li, J.; Wen, D. Realization of artificial synapses using high-performance soybean resistive memory. J. Alloys Compd. 2023, 953, 170119. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.; Li, J.; Wen, D. Full Hardware Image Encryption for Oscillating Memristor Circuits. Adv. Mater. Technol. 2023, 8, 2201540. [Google Scholar] [CrossRef]
- Vidiš, M.; Plecenik, T.; Moško, M.; Tomašec, S.; Roch, T.; Satrapinskyy, L.; Grančič, B.; Plecenik, A. Gasistor: A memristor based gas-triggered switch and gas sensor with memory. Appl. Phys. Lett. 2019, 115, 093504. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, D.; Zhao, Y.; Chiang, F.K.; Chen, P.; Guo, M.; Luo, N.; Jiang, X.; Miao, P.; Sun, Y. Evolution of Ni nanofilaments and electromagnetic coupling in the resistive switching of NiO. Nanoscale 2015, 7, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, J.; Chen, J.; Gao, L.; Yang, C.; Guo, T.; Zhao, Y.; Xiao, Y.; Wang, J.; Li, Y. High-performance memristor based on MoS2 for reliable biological synapse emulation. Mater. Today Commun. 2022, 32, 103957. [Google Scholar] [CrossRef]
- Wang, T.Y.; Meng, J.L.; Li, Q.X.; Chen, L.; Zhu, H.; Sun, Q.Q.; Ding, S.J.; Zhang, D.W. Forming-free flexible memristor with multilevel storage for neuromorphic computing by full PVD technique. J. Mater. Sci. Technol. 2021, 60, 21–26. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.; Wang, T.; Shi, X.; Liu, Y.; Zuo, Y.; Xu, L.; Wang, M.; Hu, X.; Yang, X. Robust DNA-Bridged Memristor for Textile Chips. Angew. Chem. Int. Ed. 2020, 59, 12762–12768. [Google Scholar] [CrossRef]
- Qi, M.; Cao, S.; Yang, L.; Qi, Y.; Shi, L.; Wu, Z. Uniform multilevel switching of graphene oxide-based RRAM achieved by embedding with gold nanoparticles for image pattern recognition. Appl. Phys. Lett. 2020, 116, 163503. [Google Scholar] [CrossRef]
- Ahn, M.; Park, Y.; Lee, S.H.; Chae, S.; Lee, J.; Heron, J.T.; Kioupakis, E.; Lu, W.D.; Phillips, J.D. Memristors Based on (Zr, Hf, Nb, Ta, Mo, W) High-Entropy Oxides. Adv. Electron. Mater. 2021, 7, 2001258. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, J.; Chen, P.H.; Chang, T.C.; Wu, Y.; Eshraghian, J.K.; Moon, J.; Yoo, S.; Wang, Y.H.; Chen, W.C. Adaptive synaptic memory via lithium ion modulation in RRAM devices. Small 2020, 16, 2003964. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Chen, W.; Fan, F.; Liu, G. Organic memory and memristors: From mechanisms, materials to devices. Adv. Electron. Mater. 2021, 7, 2100432. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, C.; Du, D.; Xiang, J.; Yao, S.; Hu, E.; Liu, S.; Tong, Y.; Wong, W.Y.; Zhao, Q. Donor–acceptor metallopolymers containing ferrocene for brain inspired memristive devices. Adv. Electron. Mater. 2020, 6, 2000841. [Google Scholar] [CrossRef]
- Majumdar, S.; Tan, H.; Pande, I.; Van Dijken, S. Crossover from synaptic to neuronal functionalities through carrier concentration control in Nb-doped SrTiO3-based organic ferroelectric tunnel junctions. APL Mater. 2019, 7, 091114. [Google Scholar] [CrossRef]
- Shen, Z.; Zhao, C.; Qi, Y.; Xu, W.; Liu, Y.; Mitrovic, I.Z.; Li, Y.; Zhao, C. Advances of RRAM devices: Resistive switching mechanisms, materials and bionic synaptic application. Nanomaterials 2020, 10, 1437. [Google Scholar] [CrossRef]
- Park, H.L.; Kim, M.H.; Kim, H.; Lee, S.H. Self-Selective Organic Memristor by Engineered Conductive Nanofilament Diffusion for Realization of Practical Neuromorphic System. Adv. Electron. Mater. 2021, 7, 2100299. [Google Scholar] [CrossRef]
- Mao, J.Y.; Zhou, L.; Ren, Y.; Yang, J.Q.; Chang, C.L.; Lin, H.C.; Chou, H.H.; Zhang, S.R.; Zhou, Y.; Han, S.T. A bioinspired electronic synapse using solution processable organic small molecule. J. Mater. Chem. C 2019, 7, 1491–1501. [Google Scholar] [CrossRef]
- Huang, W.Y.; Chang, Y.C.; Sie, Y.F.; Yu, C.R. Biocellulose substrate for fabricating fully biodegradable resistive random access devices. ACS Appl. Polym. Mater. 2021, 3, 4478–4484. [Google Scholar] [CrossRef]
- Sun, W.J.; Zhao, Y.Y.; Zhou, J.; Cheng, X.F.; He, J.H.; Lu, J.M. One-Step Fabrication of Bio-Compatible Coordination Complex Film on Diverse Substrates for Ternary Flexible Memory. Chem. A Eur. J. 2019, 25, 4808–4813. [Google Scholar] [CrossRef]
- Satapathi, S.; Raj, K.; Afroz, M.A. Halide-perovskite-based memristor devices and their application in neuromorphic computing. Phys. Rev. Appl. 2022, 18, 017001. [Google Scholar] [CrossRef]
- Kumar, M.; Ban, D.K.; Kim, S.M.; Kim, J.; Wong, C.P. Vertically aligned WS2 layers for high-performing memristors and artificial synapses. Adv. Electron. Mater. 2019, 5, 1900467. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Niu, Q.; Han, F.; Zhang, Y. Intrinsically ionic conductive nanofibrils for ultrathin biomemristor with low operating voltage. Sci. China Mater. 2022, 65, 3096–3104. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Li, D.; Wang, R.; Li, F.; Wu, M.; Liang, K.; Ren, H.; Zheng, X.; Guo, C. Silk protein based volatile threshold switching memristors for neuromorphic computing. Adv. Electron. Mater. 2022, 8, 2101139. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, M.; Liang, X.; Liu, C.; Saadi, M.; Chen, X.; Yao, L.; Zhang, Y.; He, N.; Hu, E. Demonstration of electronic synapses using a sericin-based biomemristor. Appl. Phys. Express 2023, 16, 031007. [Google Scholar] [CrossRef]
- Raeis-Hosseini, N.; Georgiadou, D.G.; Papavassiliou, C. High on/off ratio carbon quantum dot–chitosan biomemristors with coplanar nanogap electrodes. ACS Appl. Electron. Mater. 2022, 5, 138–145. [Google Scholar] [CrossRef]
- Saha, M.; Nawaz, S.M.; Keshari, B.K.; Mallik, A.N. Natural-casein-based biomemristor with pinched current–voltage characteristics. ACS Appl. Bio Mater. 2022, 5, 833–840. [Google Scholar] [CrossRef]
- Hussain, T.; Abbas, H.; Youn, C.; Lee, H.; Boynazarov, T.; Ku, B.; Jeon, Y.R.; Han, H.; Lee, J.H.; Choi, C. Cellulose nanocrystal based Bio-Memristor as a green artificial synaptic device for neuromorphic computing applications. Adv. Mater. Technol. 2022, 7, 2100744. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Chen, X.; Zeng, W.; Yang, J.; Zhao, Y. Sound Source Localization Based on Interaural Time Difference Using a Biologically Inspired Cochlear Model. Appl. Sci. 2021, 11, 1519. [Google Scholar]
- Wang, W.; Yin, X.; Zhao, H.; Liang, Y. Cochlear-Inspired Neural Cross-Correlogram Architecture for Sound Localization. Front. Neurosci. 2021, 15, 623316. [Google Scholar]
- Wang, S.; Song, Y.; Wang, Y.; Yang, Y.; Wei, Y.; Liu, X.; Hu, X. A Biologically Inspired Cochlear Nucleus Neural Network for Sound Source Localization Using ITD Cues. IEEE Trans. Circuits Syst. I Regul. Pap. 2020, 67, 2934–2947. [Google Scholar] [CrossRef]
- Wang, L.; Wen, D. Nonvolatile Bio-Memristor Based on Silkworm Hemolymph Proteins. Sci. Rep. 2017, 7, 17418. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Liu, Y.; Yang, H.; Guo, S.; Liu, Z.; Hu, X. The Role of Egg Albumin Membranes in the Electronic Properties of Resistive Switching Devices. Nanomaterials 2021, 11, 1943. [Google Scholar]
- Moon, T.; Soh, K.; Kim, J.S.; Yang, J.J.; Yoon, J.H. Leveraging volatile memristors in neuromorphic computing: From materials to system implementation. Mater. Horiz. 2024, 11, 4840–4866. [Google Scholar] [CrossRef]
- Shi, C.; Park, Y.; Bae, Y.; Lee, S. Multiparametric AFM insights into electron transport mechanisms in biomemristors. Mater. Today Phys. 2024, 44, 101429. [Google Scholar] [CrossRef]
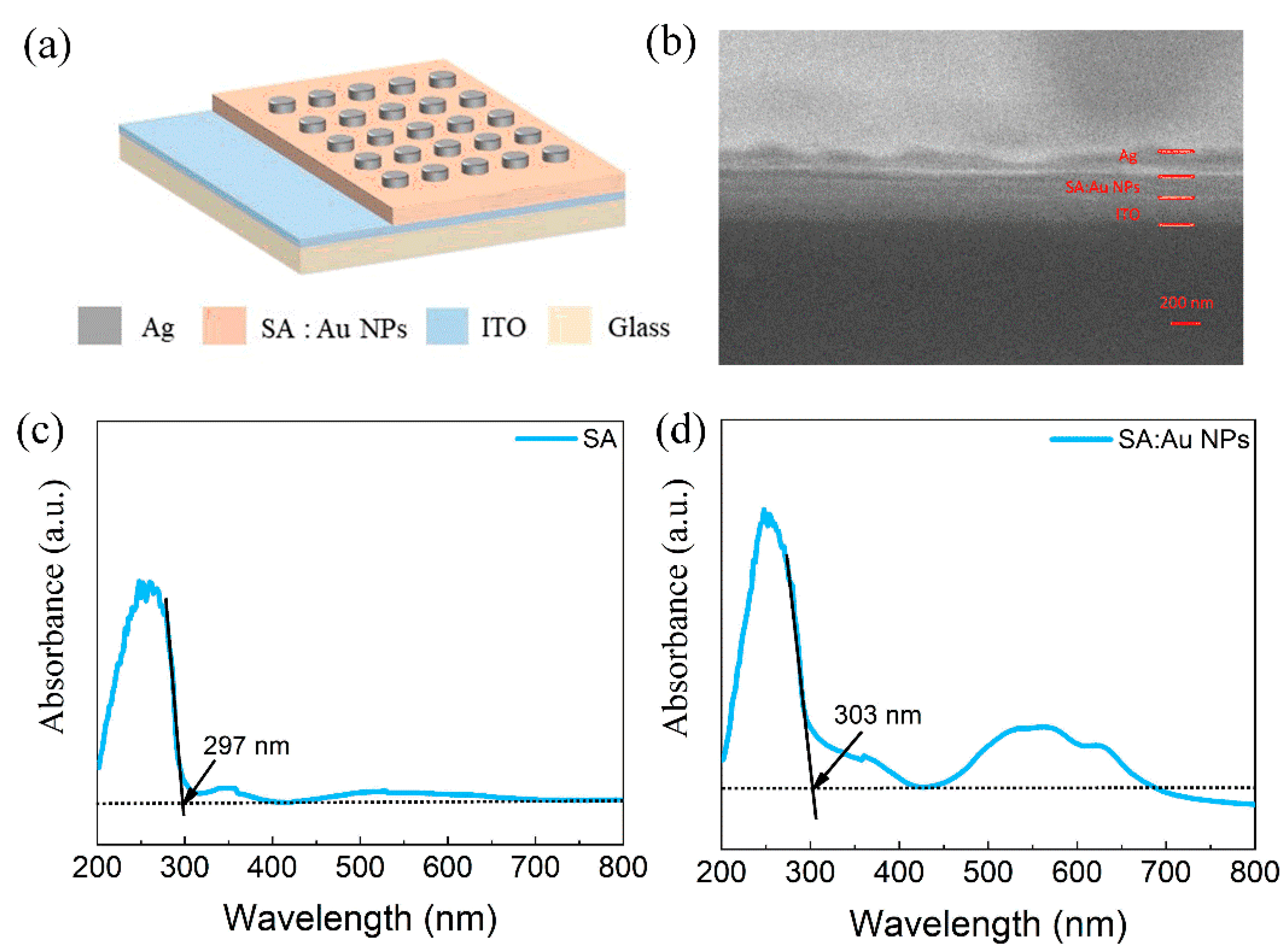
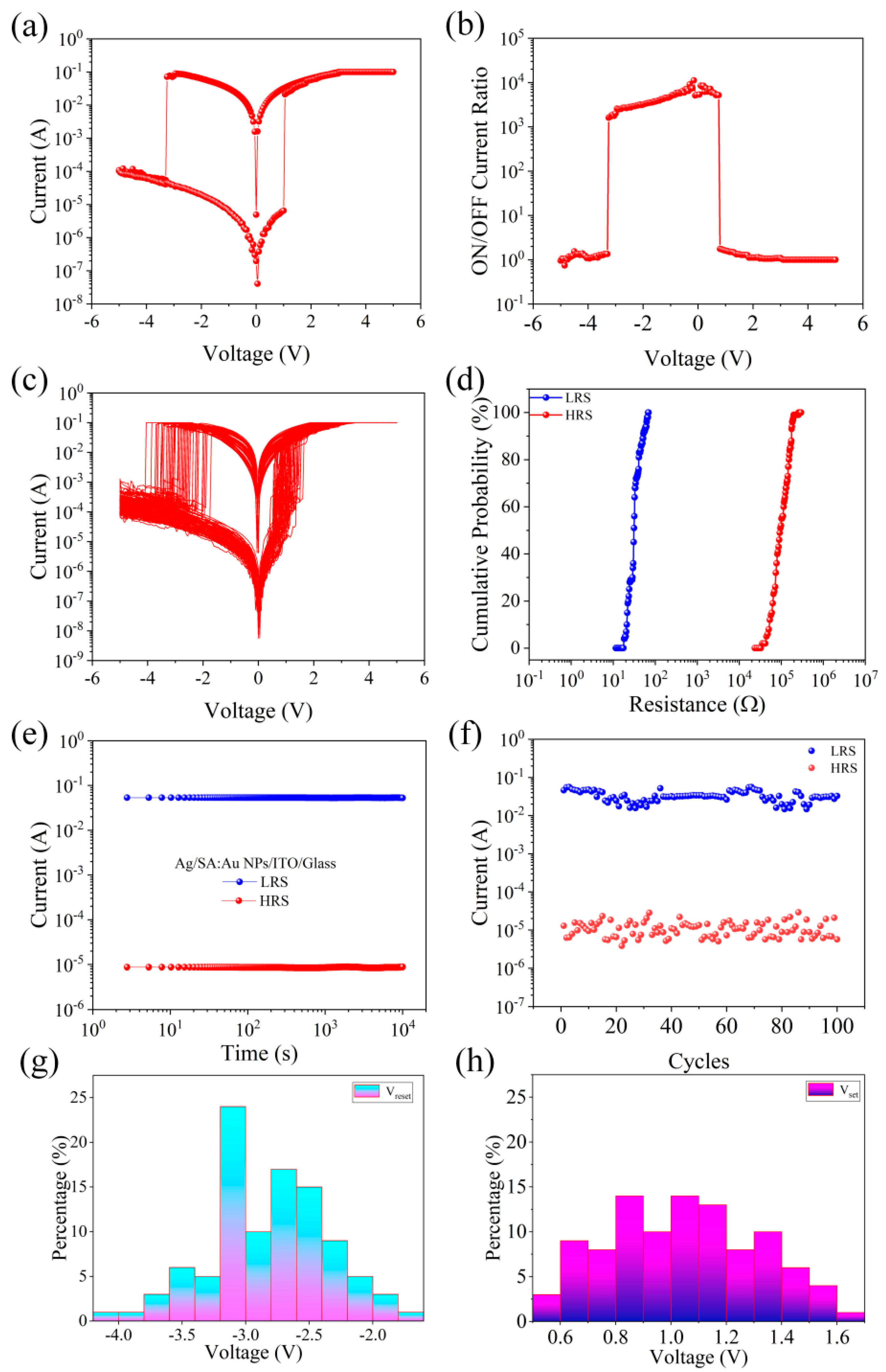
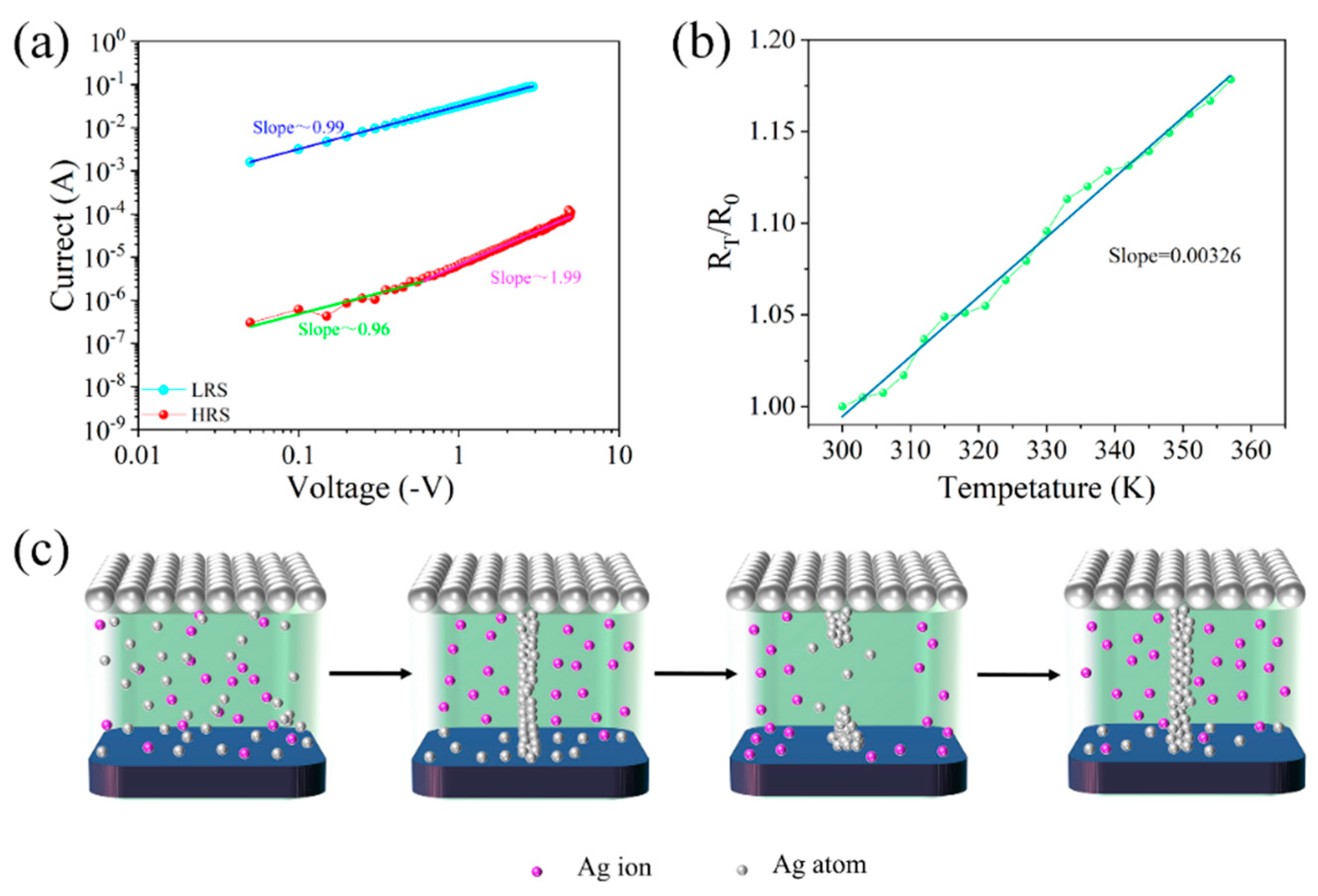

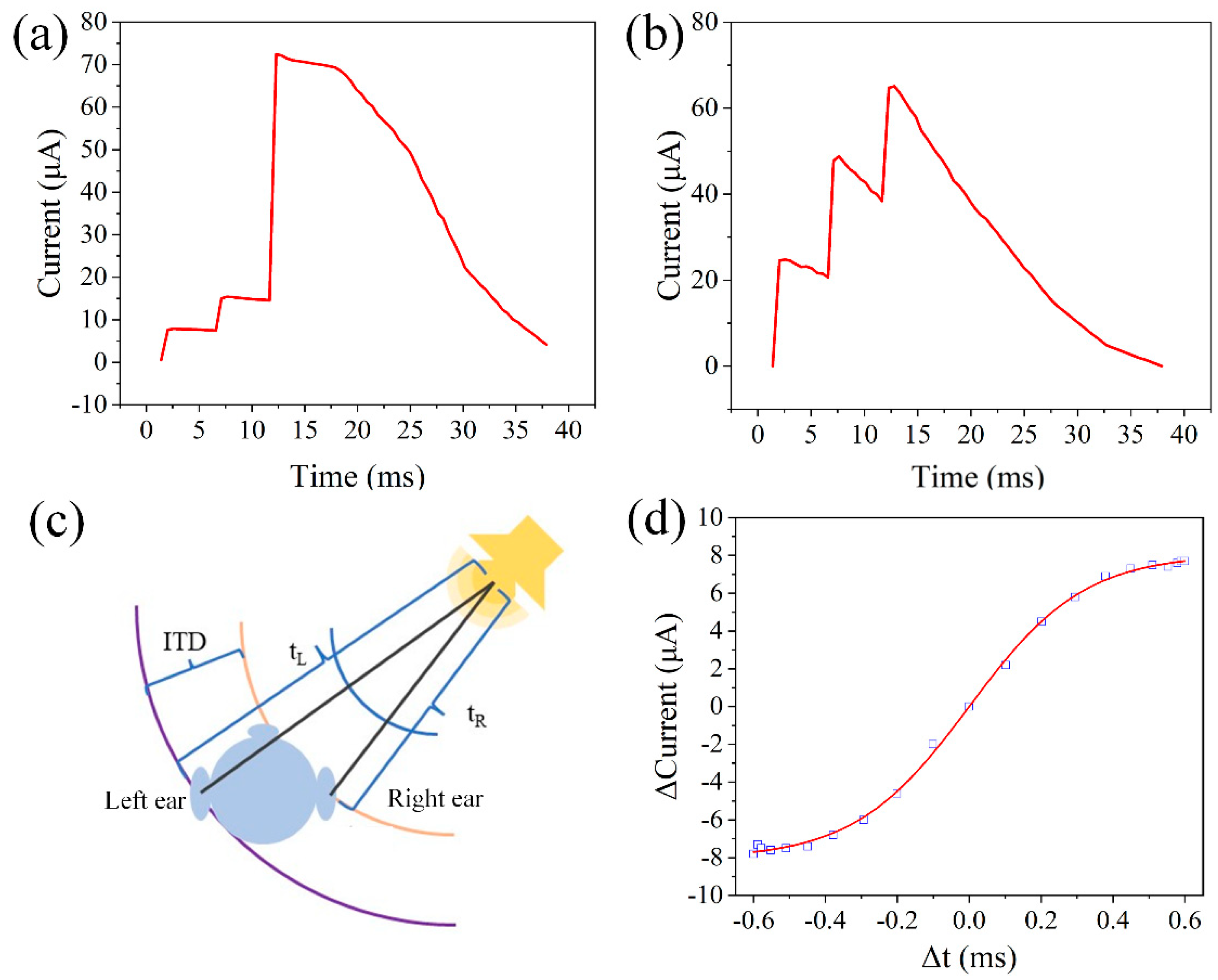
| Device Structure | Switching Current Ratio | Retention Time (s) | Durability (times) | Threshold Voltage (V) | Synaptic Behavior | References |
|---|---|---|---|---|---|---|
| Ag/SA:Au NPs/ITO | 104 | 104 | 100 | VSET = 1.02 VRESET = −2.84 | Enhancement and suppression EPSC, PPF, LTP, SRDP, STDP | This paper |
| Ag/SNFs/ITO | 102 | 105 | 180 | VSET = 0.1~0.2 VRESET = −0.2~−0.1 | “AND” and “OR” | 44 |
| Au/silk:AgNO3/Ag | 3 × 106 | 103 | 100 | / | STP PPF | 45 |
| Ag/sericin/W | 100 | / | 400 | VSET = 0.25 | SRDP STDP | 46 |
| Al/CQD−chitosan/Au | 106 | 104 | 160 | VSET = 0.75 VRESET = −1 | / | 47 |
| Al/NaCas/ITO | 20 | 105 | 180 | / | / | 48 |
| Ag/AgNPs-TCNC/FTO | 104 | 104 | 200 | VSET = 0.2 VRESET = −0.2 | LTP, LTD, EPSC, SRDP, PPF, PPD, PTP | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xie, J.; Su, W.; Du, Z.; Zhang, M. Scindapsus Aureus Resistive Random-Access Memory with Synaptic Plasticity and Sound Localization Function. Nanomaterials 2025, 15, 659. https://doi.org/10.3390/nano15090659
Wang L, Xie J, Su W, Du Z, Zhang M. Scindapsus Aureus Resistive Random-Access Memory with Synaptic Plasticity and Sound Localization Function. Nanomaterials. 2025; 15(9):659. https://doi.org/10.3390/nano15090659
Chicago/Turabian StyleWang, Lu, Jiachu Xie, Wantao Su, Zhenjie Du, and Mingzhu Zhang. 2025. "Scindapsus Aureus Resistive Random-Access Memory with Synaptic Plasticity and Sound Localization Function" Nanomaterials 15, no. 9: 659. https://doi.org/10.3390/nano15090659
APA StyleWang, L., Xie, J., Su, W., Du, Z., & Zhang, M. (2025). Scindapsus Aureus Resistive Random-Access Memory with Synaptic Plasticity and Sound Localization Function. Nanomaterials, 15(9), 659. https://doi.org/10.3390/nano15090659






