Properties of An Oral Nanoformulation of A Molecularly Dispersed Amphotericin B Comprising A Composite Matrix of Theobroma Oil and Bee’S Wax
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Lipid Nanoparticles
| AmB (mg) | 20 mL of aqueous sodium cholate solution (% w/w) | Lecithin (% w/w of total lipid) | Lipid matrix (mg) | Water (mL) | |
|---|---|---|---|---|---|
| Theobroma oil | Beeswax | ||||
| 10.0; 35.0; 50.0; 65.0 | 5.0 | 30 | 200 | 200 | 80 |
2.3. Photon Correlation Spectroscopy Analysis
2.4. Encapsulation Efficiency
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Field Emission Scanning Electron Microscopy (FESEM) and Scanning Transmission Electron Microscopy (STEM) Analysis
2.7. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Efficiency (%EE)

3.2. PCS Analysis
| Formulation | z-average diameter (nm) | PDI | ξ (mV) |
|---|---|---|---|
| Drug-free nanoparticles | 169 ± 1 | 0.215 ± 0.023 | 40.8 ± 0.9 |
| AmB-loaded nanoparticle | 222 ± 2 | 0.255 ± 0.006 | 50.3 ± 1.0 |
3.3. NTA Analysis


3.4. FESEM and STEM Analysis


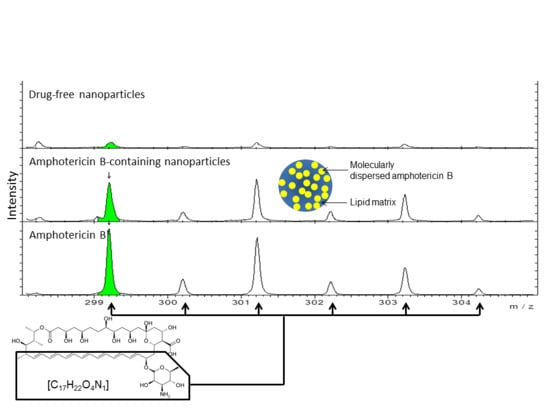
3.5. Time of Flight Secondary Ion Mass Spectroscopy (ToF-SIMS) Analysis

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Larabi, M.; Pages, N.; Pons, F.; Appel, M.; Gulik, A.; Schlatter, J.; Bouvet, S.; Barratt, G. Study of the toxicity of a new lipid complex formulation of amphotericin B. J. Antimicrob. Chemother. 2004, 53, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Larabi, M.; Yardley, V.; Loiseau, P.M.; Appel, M.; Legrand, P.; Gulik, A.; Bories, C.; Croft, S.L.; Barratt, G. Toxicity and antileishmanial activity of a new stable lipid suspension of amphotericin B. Antimicrob. Agents Chemother. 2003, 47, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Müller, R.H. Plasma protein adsorption patterns on surfaces of amphotericin B-containing fat emulsions. Int. J. Pharm. 2003, 254, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Langer, R. Oral particulate delivery: Status and future trends. Adv. Drug Deliv. Rev. 1998, 34, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.G.; Howard, K.A.; Blackball, N.W.; Thomas, N.W.; Davis, S.S.; O’Hagan, D.T. Microparticulate absorption from the rat intestine. J. Control. Release 1994, 29, 339–350. [Google Scholar] [CrossRef]
- Ebel, J.P. A method for quantifying particle absorption from the small intestine of the mouse. Pharm. Res. 1990, 7, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, J.H.; Hammond, C.J.; Meulbroek, J.A.; Staas, J.K.; Gilley, R.M.; Tice, T.R. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J. Control. Release 1990, 11, 205–214. [Google Scholar] [CrossRef]
- Florence, A.T. Nanoparticle uptake by the oral route: Fulfilling its potential? Drug Discov. Today Technol. 2005, 2, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Shakweh, M.; Besnard, M.; Nicolas, V.; Fattal, E. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by Peyer’s patches in mice. Eur. J. Pharm. Biopharm. 2005, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Kamm, W.; Breitenbach, A.; Kaiserling, E.; Xiao, J.X.; Kissel, T. Biodegradable nanoparticles for oral delivery of peptides: Is there a role for polymers to affect mucosal uptake? Eur. J. Pharm. Biopharm. 2000, 50, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Jaitley, V.; Florence, A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Billa, N.; Roberts, C.R.; Burley, J.C. Surfactant effects on the physical characteristics of amphotericin B-containing nanostructured lipid carriers. Colloids Surf. A 2010, 372, 73–79. [Google Scholar] [CrossRef]
- Tan, S.W.; Billa, N. Lipid effects on expulsion rate of amphotericin B from solid lipid nanoparticles. AAPS PharmSciTech 2014, 15, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Einstein, A. Über die von der molekularkinetischen theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten teilchen. Annalen. der Physik. 1905, 322, 549–560. (In German) [Google Scholar] [CrossRef]
- Kim, B.D.; Na, K.; Choi, H.K. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur. J. Pharm. Sci. 2005, 24, 199–205. [Google Scholar]
- Yuan, H.; Wang, L.L.; Du, Y.Z.; You, J.; Hu, F.Q.; Zeng, S. Preparation and characteristics of nanostructured lipid carriers for control-releasing progesterone by melt-emulsification. Colloids Surf. B 2007, 60, 174–179. [Google Scholar]
- Souto, R.H.; Muller, R.H. SLN and NLC for topical delivery of ketoconazole. J. Microencapsul 2005, 22, 501–510. [Google Scholar]
- Kocbek, P.; Baumgartner, S.; Kristl, J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int. J. Pharm. 2006, 312, 179–186. [Google Scholar]
- Yuan, H.; Chen, J.; Du, Y.Z.; Hu, F.Q.; Zeng, S.; Zhao, H.L. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids Surf. B 2007, 58, 157–164. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.S.W.; Billa, N.; Roberts, C.J.; Scurr, D.J. Properties of An Oral Nanoformulation of A Molecularly Dispersed Amphotericin B Comprising A Composite Matrix of Theobroma Oil and Bee’S Wax. Nanomaterials 2014, 4, 905-916. https://doi.org/10.3390/nano4040905
Tan CSW, Billa N, Roberts CJ, Scurr DJ. Properties of An Oral Nanoformulation of A Molecularly Dispersed Amphotericin B Comprising A Composite Matrix of Theobroma Oil and Bee’S Wax. Nanomaterials. 2014; 4(4):905-916. https://doi.org/10.3390/nano4040905
Chicago/Turabian StyleTan, Chloe See Wei, Nashiru Billa, Clive J. Roberts, and David J. Scurr. 2014. "Properties of An Oral Nanoformulation of A Molecularly Dispersed Amphotericin B Comprising A Composite Matrix of Theobroma Oil and Bee’S Wax" Nanomaterials 4, no. 4: 905-916. https://doi.org/10.3390/nano4040905