Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics
Abstract
:1. Introduction

2. Results and Discussion

| Sample | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Particle Size * (nm) | Zeta Potential * (mV) |
|---|---|---|---|---|---|
| Pristine IBN-4 | 345 | 7.9 | 0.75 | 271 (±3.0) | −0.1 (±0.1) |
| IBN-4 extracted | 812 | 9.3 | 1.71 | 254 (±7.6) | −10.9 (±0.4) |
| IBN-4-NH2 | 357 | 6.0 | 0.98 | 296 (±6.4) | +26.3 (±0.7) |
| IBN-4-HRP | 296 | 6.5 | 1.1 | 394 (±9.5) | +17.7 (±0.2) |


2.1. Detection of Primary Amine Groups

2.2. Properties of Immobilized HRP
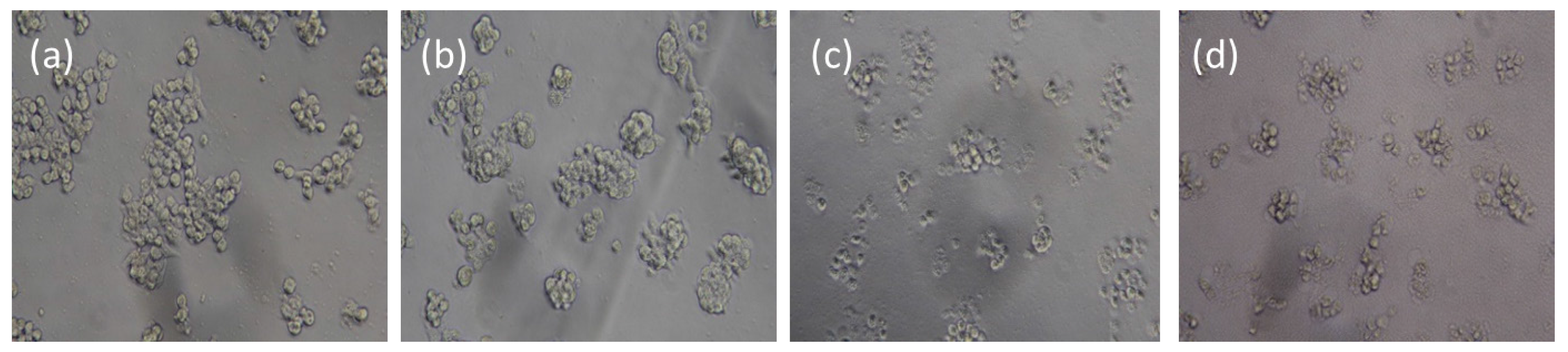
2.3. IAA-Dependent Cytotoxicity of HRP Encapsulated IBN-4





3. Experimental Section
3.1. Materials
3.2. Characterization and Instruments
3.3. Synthesis of IBN-4 Type Mesoporous Silica Nanoparticles
3.4. Synthesis of Amino-Functionalized IBN-4
3.5. Detection of Primary Amines with a Ninhydrin Test
3.6. Covalent Immobilization of HRP onto IBN-4 Materials (IBN-4-HRP)
3.7. Covalent Immobilization of HRP into IBN-4 Materials by One pot Process (IBN-4-HRP (One Pot))
3.8. HRP Enzymatic Assay
3.9. Cytotoxicity Studies
3.10. LDH Release Assay
3.11. Comet Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weyel, D.; Sedlacek, H.H.; Muller, R.; Brusselbach, S. Secreted human beta-glucuronidase: A novel tool for gene-directed enzyme prodrug therapy. Gene Ther. 2000, 7, 224–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Springer, C.J.; Niculescu-Duvaz, I.I. Antibody-directed enzyme prodrug therapy (ADEPT): A review. Adv. Drug Deliv. Rev. 1997, 26, 151–172. [Google Scholar] [PubMed]
- Springer, C.J.; Niculescu-Duvaz, I. Prodrug-activating systems in suicide gene therapy. J. Clin. Investig. 2000, 105, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Connors, T.A. The choice of prodrugs for gene directed enzyme prodrug therapy of cancer. Gene Ther. 1995, 2, 702–709. [Google Scholar] [PubMed]
- Hamstra, D.A.; Rehemtulla, A. Toward an enzyme/prodrug strategy for cancer gene therapy: Endogenous activation of carboxypeptidase a mutants by the PACE/Furin family of propeptidases. Hum. Gene Ther. 1999, 10, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Rigg, A.; Sikora, K. Genetic prodrug activation therapy. Mol. Med. Today 1997, 3, 359–366. [Google Scholar] [CrossRef]
- Rainov, N.G.; Dobberstein, K.U.; Sena-Esteves, M.; Herrlinger, U.; Kramm, C.M.; Philpot, R.M.; Hilton, J.; Chiocca, E.A.; Breakefield, X.O. New prodrug activation gene therapy for cancer using cytochrome P450 4B1 and 2-aminoanthracene/4-ipomeanol. Hum. Gene Ther. 1998, 9, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.C.; Khakhar, A.; Eshleman, J.R.; Ostermeier, M. Advancements in the development of HIF-1ɑ-activated protein switches for use in enzyme prodrug therapy. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kale, V.; Chen, M. Gene-directed enzyme prodrug therapy. AAPS J. 2014, 17, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Bhuniya, S.; Lee, H.; Kim, H.M.; Cheong, C.; Maiti, S.; Hong, K.S.; Kim, J.S. An activatable prodrug for the treatment of metastatic tumors. J. Am. Chem. Soc. 2014, 136, 13888–13894. [Google Scholar] [CrossRef] [PubMed]
- Bagshawe, K.D. Antibody-directed enzyme prodrug therapy (ADEPT) for cancer. Expert Rev. Anticancer Ther. 2006, 6, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.B.; Burke, P.J.; Somani, H.H.; Friedlos, F.; Knox, R.J. Self-immolative prodrugs: Candidates for antibody-directed enzyme prodrug therapy in conjunction with a nitroreductase enzyme. J. Med. Chem. 1994, 37, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Napier, M.P.; Sharma, S.K.; Springer, C.J.; Bagshawe, K.D.; Green, A.J.; Martin, J.; Stribbling, S.M.; Cushen, N.; O’Malley, D.; Begent, R.H. Antibody-directed enzyme prodrug therapy: Efficacy and mechanism of action in colorectal carcinoma. Clin. Cancer Res. 2000, 6, 765–772. [Google Scholar] [PubMed]
- Palmer, D.H.; Mautner, V.; Mirza, D.; Oliff, S.; Gerritsen, W.; van der Sijp, J.R.; Hubscher, S.; Reynolds, G.; Bonney, S.; Rajaratnam, R.; et al. Virus-directed enzyme prodrug therapy: Intratumoral administration of a replication-deficient adenovirus encoding nitroreductase to patients with resectable liver cancer. J. Clin. Oncol. 2004, 22, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Tychopoulos, M.; Corcos, L.; Genne, P.; Beaune, P.; de Waziers, I. A virus-directed enzyme prodrug therapy (VDEPT) strategy for lung cancer using a CYP2B6/NADPH-cytochrome P450 reductase fusion protein. Cancer Gene Ther. 2005, 12, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Garnier, P.; Wang, X.T.; Robinson, M.A.; van Kasteren, S.; Perkins, A.C.; Frier, M.; Fairbanks, A.J.; Davis, B.G. Lectin-directed enzyme activated prodrug therapy (LEAPT): Synthesis and evaluation of rhamnose-capped prodrugs. J. Drug Target. 2010, 18, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Liu, Z.; Thomale, J.; Dai, H.; Lippard, S.J. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 2008, 130, 11467–11476. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.J.; Jagtenberg, J.C.; Haisma, H.J.; Storm, G. Liposome-mediated targeting of enzymes to cancer cells for site-specific activation of prodrugs: Comparison with the corresponding antibody-enzyme conjugate. Pharm. Res. 2003, 20, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoeds, M.H.; Haisma, H.J.; Belliot, S.O.; Smit, R.H.; Crommelin, D.J.; Storm, G. Immunoliposomes as enzyme-carriers (immuno-enzymosomes) for antibody-directed enzyme prodrug therapy (ADEPT): Optimization of prodrug activating capacity. Pharm. Res. 1996, 13, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Koning, G.A.; Morselt, H.W.; Velinova, M.J.; Donga, J.; Gorter, A.; Allen, T.M.; Zalipsky, S.; Kamps, J.A.; Scherphof, G.L. Selective transfer of a lipophilic prodrug of 5-fluorodeoxyuridine from immunoliposomes to colon cancer cells. Biochim. Biophys. Acta 1999, 1420, 153–167. [Google Scholar] [CrossRef]
- Vingerhoeds, M.H.; Haisma, H.J.; van Muijen, M.; van de Rijt, R.B.; Crommelin, D.J.; Storm, G. A new application for liposomes in cancer therapy. Immunoliposomes bearing enzymes (immuno-enzymosomes) for site-specific activation of prodrugs. FEBS Lett. 1993, 336, 485–490. [Google Scholar] [CrossRef]
- Eshita, Y.; Higashihara, J.; Onishi, M.; Mizuno, M.; Yoshida, J.; Takasaki, T.; Kubota, N.; Onishi, Y. Mechanism of introduction of exogenous genes into cultured cells using DEAE-Dextran-MMA graft copolymer as non-viral gene carrier. Molecules 2009, 14, 2669–2683. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kolishetti, N.; Lippard, S.J.; Farokhzad, O.C. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Duan, S.; Zeng, X.; Liu, C.; Davies, N.M.; Li, B.; Forrest, M.L. Prodrug strategy for psma-targeted delivery of TGX-221 to prostate cancer cells. Mol. Pharm. 2012, 9, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Andresen, T.L.; Davidsen, J.; Hoyrup, P.; Shnyder, S.D.; Bibby, M.C.; Gill, J.H.; Jorgensen, K. Secretory phospholipase A2 as a tumor-specific trigger for targeted delivery of a novel class of liposomal prodrug anticancer etherlipids. Mol. Cancer Ther. 2004, 3, 1451–1458. [Google Scholar] [PubMed]
- Zhu, S.; Lansakara, P.D.; Li, X.; Cui, Z. Lysosomal delivery of a lipophilic gemcitabine prodrug using novel acid-sensitive micelles improved its antitumor activity. Bioconjug. Chem. 2012, 23, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Drbohlavova, J.; Chomoucka, J.; Adam, V.; Ryvolova, M.; Eckschlager, T.; Hubalek, J.; Kizek, R. Nanocarriers for anticancer drugs—New trends in nanomedicine. Curr. Drug Metab. 2013, 14, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Pietersen, L.K.; Govender, P.; Kruger, H.G.; Maguire, G.E.; Govender, T. Enzymatic activation of a peptide functionalised gold nanoparticle system for prodrug delivery. J. Nanosci. Nanotechnol. 2011, 11, 3075–3083. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhar, S.; Daniel, W.L.; Giljohann, D.A.; Mirkin, C.A.; Lippard, S.J. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J. Am. Chem. Soc. 2009, 131, 14652–14653. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fang, G.; Wang, X.; Zeng, F.; Xiang, Y.; Wu, S. Targeted anticancer prodrug with mesoporous silica nanoparticles as vehicles. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-M.; Kuthati, Y.; Kankala, R.K.; Wei, P.-R.; Weng, C.-F.; Liu, C.-L.; Sung, P.-J.; Mou, C.-Y.; Lee, C.-H. Layered double hydroxide nanoparticles to enhance organ-specific targeting and the anti-proliferative effect of cisplatin. J. Mater. Chem. B 2015, 3, 3447–3458. [Google Scholar] [CrossRef]
- Gwenin, V.V.; Gwenin, C.D.; Kalaji, M. Colloidal gold modified with a genetically engineered nitroreductase: Toward a novel enzyme delivery system for cancer prodrug therapy. Langmuir 2011, 27, 14300–14307. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shrestha, T.B.; Basel, M.T.; Dani, R.K.; Seo, G.M.; Balivada, S.; Pyle, M.M.; Prock, H.; Koper, O.B.; Thapa, P.S.; et al. Magnetic-Fe/Fe3O4-nanoparticle-bound SN38 as carboxylesterase-cleavable prodrug for the delivery to tumors within monocytes/macrophages. Beilstein J. Nanotechnol. 2012, 3, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Cude, M.P.; Gwenin, C.D. Development of gold coated superparamagnetic iron oxide nanoparticles for nitroreductase delivery. ECS Trans. 2011, 33, 79–89. [Google Scholar]
- Chiu, Y.-R.; Ho, W.-J.; Chao, J.-S.; Yuan, C.-J. Enzyme-encapsulated silica nanoparticle for cancer chemotherapy. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar]
- Kankala, R.K.; Kuthati, Y.; Liu, C.-L.; Mou, C.-Y.; Lee, C.-H. Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ROS. RSC Adv. 2015, 5, 86072–86081. [Google Scholar] [CrossRef]
- Kankala, R.K.; Kuthati, Y.; Liu, C.-L.; Lee, C.-H. Hierarchical coated metal hydroxide nanoconstructs as potential controlled release carriers of photosensitizer for skin melanoma. RSC Adv. 2015, 5, 42666–42680. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Lee, C.-H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112–113, 100–116. [Google Scholar] [CrossRef]
- Giri, S.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanomaterial-based biotechnological and biomedical delivery systems. Nanomedicine 2007, 2, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Gao, L.; Wang, J.; Shokouhimehr, M.; Liu, J.; Yu, Y.; Hackett, M.J.; So, P.-K.; Zheng, B.; Yao, Z.; et al. A general strategy for site-directed enzyme immobilization by using NiO nanoparticle decorated mesoporous silica. Chem. Eur. J. 2014, 20, 7916–7921. [Google Scholar] [CrossRef] [PubMed]
- Maddala, S.P.; Mastroianni, G.; Velluto, D.; Sullivan, A.C. Intracellular delivery of BSA by phosphonate@silica nanoparticles. J. Mater. Chem. B 2015, 3, 6057–6070. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Lin, V.S.Y.; Slowing, I.I.; Trewyn, B.G. Luciferase and luciferin co-immobilized mesoporous silica nanoparticle materials for intracellular biocatalysis. J. Am. Chem. Soc. 2011, 133, 18554–18557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Chen, C.-T.; Hung, Y.; Chou, C.-M.; Liu, T.-P.; Liang, M.-R.; Chen, C.-T.; Mou, C.-Y. A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: Superoxide dismutase. J. Am. Chem. Soc. 2013, 135, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Caruso, F. Mesoporous silica spheres as supports for enzyme immobilization and encapsulation. Chem. Mater. 2005, 17, 953–961. [Google Scholar] [CrossRef]
- Lee, C.H.; Lang, J.; Yen, C.W.; Shih, P.C.; Lin, T.S.; Mou, C.Y. Enhancing stability and oxidation activity of cytochrome c by immobilization in the nanochannels of mesoporous aluminosilicates. J. Phys. Chem. B 2005, 109, 12277–12286. [Google Scholar] [CrossRef] [PubMed]
- Méndez, J.; Morales Cruz, M.; Delgado, Y.; Figueroa, C.M.; Orellano, E.A.; Morales, M.; Monteagudo, A.; Griebenow, K. Delivery of chemically glycosylated cytochrome c immobilized in mesoporous silica nanoparticles induces apoptosis in HeLa cancer cells. Mol. Pharm. 2014, 11, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Méndez, J.; Monteagudo, A.; Griebenow, K. Stimulus-responsive controlled release system by covalent immobilization of an enzyme into mesoporous silica nanoparticles. Bioconj. Chem. 2012, 23, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J. Am. Chem. Soc. 2007, 129, 8845–8849. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, C.W.; Lee, H.J.; Choi, J.H.; Lee, S.G.; Yun, Y.P.; Kwon, I.C.; Lee, S.J.; Jeong, S.Y.; Lee, S.C. A mesoporous silica nanoparticle with charge-convertible pore walls for efficient intracellular protein delivery. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Urata, C.; Ujiie, H.; Yamauchi, Y.; Kuroda, K. Preparation of aqueous colloidal mesostructured and mesoporous silica nanoparticles with controlled particle size in a very wide range from 20 nm to 700 nm. Nanoscale 2013, 5, 6145–6153. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhou, L.; Zhang, J.; Yuan, P.; Thorn, P.; Gu, W.; Yu, C. A simple approach to prepare monodisperse mesoporous silica nanospheres with adjustable sizes. J. Colloid Interface Sci. 2012, 376, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Liao, W.N.; Chen, L.M.; Lee, C.H. pH-controllable release using functionalized mesoporous silica nanoparticles as an oral drug delivery system. J. Mater. Chem. 2011, 21, 7130–7137. [Google Scholar] [CrossRef]
- Lee, C.H.; Lo, L.W.; Mou, C.Y.; Yang, C.S. Synthesis and characterization of positive-charge functionalized mesoporous silica nanoparticles for oral drug delivery of an anti-inflammatory drug. Adv. Funct. Mater. 2008, 18, 3283–3292. [Google Scholar] [CrossRef]
- Lee, C.-H.; Cheng, S.-H.; Huang, I.P.; Souris, J.S.; Yang, C.-S.; Mou, C.-Y.; Lo, L.-W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. 2010, 122, 8390–8395. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Lin, S.-X.; Weng, C.-F.; Lee, C.-H. pH-Triggered controllable release of silver-indole-3 acetic acid complexes from mesoporous silica nanoparticles (IBN-4) for effectively killing malignant bacteria. Mol. Pharm. 2015, 12, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Balas, F.; Manzano, M.; Vallet-Regí, M. Novel method to synthesize ordered mesoporous silica with high surface areas. Solid State Sci. 2008, 10, 408–415. [Google Scholar] [CrossRef]
- Kuthati, Y.; Sung, P.J.; Weng, C.F.; Mou, C.Y.; Lee, C.H. Functionalization of mesoporous silica nanoparticles for targeting, biocompatibility, combined cancer therapies and theragnosis. J. Nanosci. Nanotechnol. 2013, 13, 2399–2430. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Catalytic activity in organic solvents and stability of immobilized enzymes depend on the pore size and surface characteristics of mesoporous silica. Chem. Mater. 2000, 12, 3301–3305. [Google Scholar] [CrossRef]
- Díaz, J.F.; Balkus, K.J., Jr. Enzyme immobilization in MCM-41 molecular sieve. J. Mol. Catal. B 1996, 2, 115–126. [Google Scholar] [CrossRef]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Immobilized enzymes in ordered mesoporous silica materials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater. 2001, 44–45, 755–762. [Google Scholar] [CrossRef]
- Lee, C.H.; Wong, S.T.; Lin, T.S.; Mou, C.Y. Characterization and biomimetic study of a hydroxo-bridged dinuclear phenanthroline cupric complex encapsulated in mesoporous silica: Models for catechol oxidase. J. Phys. Chem. B 2005, 109, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Siefker, J.; Karande, P.; Coppens, M.O. Packaging biological cargoes in mesoporous materials: Opportunities for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Marina, M.-C.; Montserrat, C.; Maria, V.-R. Smart mesoporous nanomaterials for antitumour therapy. Nanomaterials 2015, 5, 1906–1937. [Google Scholar]
- Kim, D.-S.; Jeon, S.-E.; Park, K.-C. Oxidation of indole-3-acetic acid by horseradish peroxidase induces apoptosis in G361 human melanoma cells. Cell. Signal. 2004, 16, 81–88. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, K.; Zhu, X.; Li, Y.; Wei, J.; Zhao, W.; Liu, C.; Shi, J. Sub-150 nm mesoporous silica nanoparticles with tunable pore sizes and well-ordered mesostructure for protein encapsulation. J. Colloid Interface Sci. 2013, 407, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Schmalenberg, K.E.; Frauchiger, L.; Nikkhouy-Albers, L.; Uhrich, K.E. Cytotoxicity of a unimolecular polymeric micelle and its degradation products. Biomacromolecules 2001, 2, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Grabinski, C.; Schrand, A.; Murdock, R.; Wang, W.; Gu, B.; Schlager, J.; Hussain, S. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J. Nanopart. Res. 2009, 11, 15–24. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.; Hung, Y.; Mou, C. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Smeller, L.; Meersman, F.; Fidy, J.; Heremans, K. High-pressure FTIR study of the stability of horseradish peroxidase. Effect of heme substitution, ligand binding, Ca++ removal, and reduction of the disulfide bonds. Biochemistry 2003, 42, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Lison, D.; Thomassen, L.C.J.; Rabolli, V.; Gonzalez, L.; Napierska, D.; Seo, J.W.; Kirsch-Volders, M.; Hoet, P.; Kirschhock, C.E.A.; Martens, J.A. Nominal and effective dosimetry of silica nanoparticles in cytotoxicity assays. Toxicol. Sci. 2008, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.M.; Oh, M.H.; Kim, S.Y.; Li, H.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, W.Y.; Kim, D.S. Indole-3-acetic acid/horseradish peroxidase induces apoptosis in TCCSUP human urinary bladder carcinoma cells. Pharmazie 2010, 65, 122–126. [Google Scholar] [PubMed]
- Han, Y.; Ying, J.Y. Generalized fluorocarbon-surfactant-mediated synthesis of nanoparticles with various mesoporous structures. Angew. Chem. Int. Ed. 2005, 44, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, D.; Huang, H.; Huang, B.; Cai, H. Mesoporous silicas prepared by ammonium perchlorate oxidation and theirs application in the selective adsorption of high explosives. Microporous Mesoporous Mater. 2013, 168, 46–50. [Google Scholar] [CrossRef]
- Taylor, I.; Howard, A.G. Measurement of primary amine groups on surface-modified silica and their role in metal binding. Anal. Chim. Acta 1993, 271, 77–82. [Google Scholar] [CrossRef]
- Jung, D.; Streb, C.; Hartmann, M. Covalent anchoring of chloroperoxidase and glucose oxidase on the mesoporous molecular sieve SBA-15. Int. J. Mol. Sci. 2010, 11, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.I.; Kim, J.; Lee, J.; Jia, H.; Na, H.B.; Youn, J.K.; Kwak, J.H.; Dohnalkova, A.; Grate, J.W.; Wang, P.; et al. Crosslinked enzyme aggregates in hierarchically-ordered mesoporous silica: A simple and effective method for enzyme stabilization. Biotechnol. Bioeng. 2007, 96, 210–218. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, B.-Y.; Kuthati, Y.; Kankala, R.K.; Kankala, S.; Deng, J.-P.; Liu, C.-L.; Lee, C.-H. Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics. Nanomaterials 2015, 5, 2169-2191. https://doi.org/10.3390/nano5042169
Hung B-Y, Kuthati Y, Kankala RK, Kankala S, Deng J-P, Liu C-L, Lee C-H. Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics. Nanomaterials. 2015; 5(4):2169-2191. https://doi.org/10.3390/nano5042169
Chicago/Turabian StyleHung, Bau-Yen, Yaswanth Kuthati, Ranjith Kumar Kankala, Shravankumar Kankala, Jin-Pei Deng, Chen-Lun Liu, and Chia-Hung Lee. 2015. "Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics" Nanomaterials 5, no. 4: 2169-2191. https://doi.org/10.3390/nano5042169
APA StyleHung, B.-Y., Kuthati, Y., Kankala, R. K., Kankala, S., Deng, J.-P., Liu, C.-L., & Lee, C.-H. (2015). Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics. Nanomaterials, 5(4), 2169-2191. https://doi.org/10.3390/nano5042169






