Nanostructured Electrospun Hybrid Graphene/Polyacrylonitrile Yarns
Abstract
:1. Introduction
2. Results
2.1. Rheological Behavior of LCGO and PAN/LCGO Composite Suspensions
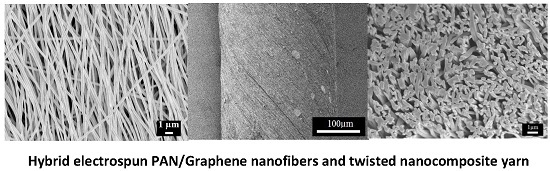
2.2. As-Prepared Hybrid Electrospun Nanofibers
2.3. Morphology of PAN/LCGO Nanofibrous Mats and Twisted Yarns
2.4. Raman Spectroscopy of PAN/LCGO Nanofibrous Twisted Yarns
2.5. Differential Scanning Calorimetry Thermograms of PAN/LCGO Nanofibrous Twisted Yarns
2.6. Mechanical Properties of PAN/LCGO Nanofibrous Twisted Yarns
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Q.; Du, Y.; Feng, Q.; Huang, F.; Lu, K.; Liu, J.; Wei, Q. Nanostructures and surface nanomechanical properties of polyacrylonitrile/graphene oxide composite nanofibers by electrospinning. J. Appl. Polym. Sci. 2013, 128, 1152–1157. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Papkov, D.; Goponenko, A.; Compton, O.C.; An, Z.; Moravsky, A.; Li, X.-Z.; Nguyen, S.T.; Dzenis, Y.A. Improved graphitic structure of continuous carbon nanofibers via graphene oxide templating. Adv. Funct. Mater. 2013, 23, 5673–5770. [Google Scholar] [CrossRef]
- Xiang, C.; Behabtu, N.; Liu, Y.; Chae, H.G.; Young, C.C.; Genorio, B.; Tsentalovich, D.E.; Zhang, C.; Kosynkin, D.V.; Lomeda, J.R.; et al. Graphene nanoribbons as an advanced precursor for making carbon fiber. ACS Nano 2013, 7, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.L.; Venton, B.J. Carbon-fiber microelectrodes for in vivo applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dufficy, M.K.; Khan, S.A.; Fedkiw, P.S. Hierarchical Graphene-Containing Carbon Nanofibers for Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2016, 8, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yue, D.; Xu, Z.-W.; Liang, G.; Zhang, Y.; Zhang, J.-F.; Zhang, X.; Wang, Z. Fabrication, mechanical properties, and biocompatibility of reduced graphene oxide-reinforced nanofiber mats. RSC Adv. 2014, 4, 35035–35041. [Google Scholar] [CrossRef]
- Zussman, E.; Chen, X.; Ding, W.; Calabri, L.; Dikin, D.A.; Quintana, J.P.; Ruoff, R.S. Mechanical and structural characterization of electrospun PAN-derived carbon nanofibers. Carbon 2005, 43, 2175–2185. [Google Scholar] [CrossRef]
- Gu, S.-Y.; Wu, Q.-L.; Ren, J. Preparation and surface structures of carbon nanofibers produced from electrospun PAN precursors. New Carbon Mater. 2008, 23, 171–176. [Google Scholar] [CrossRef]
- Kaerkitcha, N.; Chuangchote, S.; Sagawa, T. Control of physical properties of carbon nanofibers obtained from coaxial electrospinning of PMMA and PAN with adjustable inner/outer nozzle-ends. Nanoscale Res. Lett. 2016, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouya, F.; Tavanai, H.; Morshed, M.; Ghiaci, M. The formation of titanium dioxide crystallite nanoparticles during activation of PAN nanofibers containing titanium isopropoxide. J. Nanopart. Res. 2012, 14, 1074. [Google Scholar] [CrossRef]
- Gu, S.Y.; Ren, J.; Wu, Q.L. Preparation and structures of electrospun PAN nanofibers as a precursor of carbon nanofibers. Synth. Met. 2005, 155, 157–161. [Google Scholar] [CrossRef]
- Hou, H.; Ge, J.J.; Zeng, J.; Li, Q.; Reneker, D.H.; Greiner, A.; Cheng, S.Z.D. Electrospun Polyacrylonitrile Nanofibers Containing a High Concentration of Well-Aligned Multiwall Carbon Nanotubes. Chem. Mater. 2005, 17, 967–973. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sokhi, S.; Balomajumder, C.; Satapathi, S. Reusable graphene oxide nanofibers for enhanced photocatalytic activity: A detailed mechanistic study. J. Mater. Sci. 2017, 52, 5390–5403. [Google Scholar] [CrossRef]
- Matsumoto, H.; Imaizumi, S.; Konosu, Y.; Ashizawa, M.; Minagawa, M.; Tanioka, A.; Lu, W.; Tour, J.M. Electrospun composite nanofiber yarns containing oriented graphene nanoribbons. ACS Appl. Mater. Interfaces 2013, 5, 6225–6231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, G.; Zhang, J.; Huang, F.; Lu, K.; Wei, Q. PAN Nanofibers Reinforced with MMT/GO Hybrid Nanofillers. J. Nanomater. 2014, 2014, 298021. [Google Scholar] [CrossRef]
- Ge, J.J.; Hou, H.; Li, Q.; Graham, M.J.; Greiner, A.; Reneker, D.H.; Harris, F.W.; Cheng, S.Z.D. Assembly of Well-Aligned Multiwalled Carbon Nanotubes in Confined Polyacrylonitrile Environments: Electrospun Composite Nanofiber Sheets. J. Am. Chem. Soc. 2004, 126, 15754–15761. [Google Scholar] [CrossRef] [PubMed]
- Go, D.; Lott, P.; Stollenwerk, J.; Thomas, H.; Möller, M.; Kuehne, A.J.C. Laser Carbonization of PAN-Nanofiber Mats with Enhanced Surface Area and Porosity. ACS Appl. Mater. Interfaces 2016, 8, 28412–28417. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lu, Y.; Shao, G.; Zeng, F.; Wu, Q. Preparation of polyacrylonitrile/graphene oxide by in situ polymerization. Polym. Int. 2012, 61, 1394–1399. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Lu, Y.; Zang, J.; Jiang, M.; Kim, D.; Zhang, X. Highly porous polyacrylonitrile/graphene oxide membrane separator exhibiting excellent anti-self-discharge feature for high-performance lithium–sulfur batteries. Carbon 2016, 101, 272–280. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-Dimensional (2D) Nanomaterials towards Electrochemical Nanoarchitectonics in Energy-Related Applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Mannix, A.J.; Kiraly, B.; Hersam, M.C.; Guisinger, N.P. Synthesis and chemistry of elemental 2D materials. Nat. Rev. Chem. 2017, 1, 0014. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cheng, Q.-Y.; Wu, H.; Wei, Z.; Han, B.-H. Graphene oxide-based benzimidazole-crosslinked networks for high-performance supercapacitors. Nanoscale 2013, 5, 8367–8374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, C. Graphene fiber: A new trend in carbon fibers. Mater. Today 2015, 18, 480–492. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.-J.; Kim, D.-H.; Ku, B.-C.; Joh, H.-I. Synthesisandpropertiesofthermallyreducedgraphene oxide/polyacrylonitrilecomposites. J. Phys. Chem. Solids 2012, 73, 741–743. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Oh, B.-Y.; Seo, D.-S.; Li, X. Super-fast switching of liquid crystals sandwiched between highly conductive graphene oxide/dimethyl sulfate doped PEDOT:PSS composite layers. J. Appl. Phys. 2016, 119, 194505. [Google Scholar] [CrossRef]
- Lin, F.; Tong, X.; Wang, Y.; Bao, J.; Wang, Z. Graphene oxide liquid crystals: Synthesis, phase transition, rheological property, and applications in optoelectronics and display. Nanoscale Res. Lett. 2015, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Choi, B.G.; Hong, W.H.; Jang, S.-Y. Controlled assembly of graphene oxide nanosheets within one-dimensional polymer nanostructure. J. Coll. Interface Sci. 2013, 406, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Hu, X.; Gao, C. Lyotropic liquid crystal of polyacrylonitrile-grafted graphene oxide and its assembled continuous strong nacre-mimetic fibers. Macromolecules 2013, 46, 6931–6941. [Google Scholar] [CrossRef]
- Ke, H.; Pang, Z.; Xu, Y.; Chen, X.; Fu, J.; Cai, Y.; Huang, F.; Wei, Q. Graphene oxide improved thermal and mechanical properties of electrospun methyl stearate/polyacrylonitrile form-stable phase change composite nanofibers. J. Therm. Anal. Calorim. 2014, 117, 109–122. [Google Scholar] [CrossRef]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Konstantinov, K.; Razal, J.M.; Moulton, S.E.; Wallace, G.G. Formation and processability of liquid crystalline dispersions of graphene oxide. Mater. Horiz. 2014, 1, 87–91. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Li, M.; Gosselink, D.; Zhang, Y.; Chen, P. A sulfurepolyacrylonitrile/graphene composite cathode for lithium batteries with excellent cyclability. J. Power Source 2014, 252, 107–112. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.-F. Graphene-beaded carbon nanofibers for use in supercapacitor electrodes: Synthesis and electrochemical characterization. J. Power Source 2013, 222, 410–416. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Pan, X.; Jin, Y.; Lu, W.; Ding, D.; Guo, Q. Graphene oxide/polyacrylonitrile fiber hierarchical-structured membrane for ultra-fast microfiltration of oil-water emulsion. Chem. Eng. J. 2017, 307, 643–649. [Google Scholar] [CrossRef]
- Chien, A.-T.; Liu, H.C.; Newcomb, B.A.; Xiang, C.; Tour, J.M.; Kumar, S. Polyacrylonitrile Fibers Containing Graphene Oxide Nanoribbons. ACS Appl. Mater. Interfaces 2015, 7, 5281–5288. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, Y.; Qi, K.; Wang, L.; Li, P.; Cui, S. Continuous twisted nanofiber yarns fabricated by double conjugate electrospinning. Fibers Polym. 2013, 14, 1857–1863. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Bhandari, S.; Biradar, A.M.; Reddy, G.B.; Pasricha, R. Exfoliation of graphene oxide and its application in improving the electro-optical response of ferroelectric liquid crystal. J. Appl. Phys. 2015, 118, 114904. [Google Scholar] [CrossRef]
- Yao, B.; Chen, J.; Huang, L.; Zhou, Q.; Shi, G. Base-induced liquid crystals of graphene oxide for preparing elastic graphene foams with long-range ordered microstructures. Adv. Mater. 2016, 28, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Konstantinov, K.; Moulton, S.E.; Razal, J.M.; Wallace, G.G. Organic solvent-based graphene oxide liquid crystals: A facile route toward the next generation of self-assembled layer-by-layer multifunctional 3D architectures. ACS Nano 2013, 7, 3981–3990. [Google Scholar] [CrossRef] [PubMed]







| Sample | Solvent | PAN (wt %) | LCGO (wt %) | GO (in NFs Mats) % | Average NFs Diameter (nm) |
|---|---|---|---|---|---|
| PAN-A | DMF | 10 | ---- | --- | 252 |
| PAN/LCGO-B | DMF | 10 | 0.01 | 0.099 | 251 |
| PAN/LCGO-C | DMF | 10 | 0.031 | 0.309 | 311 |
| PAN/LCGO-D | DMF | 10 | 0.157 | 1.546 | 345 |
| PAN/LCGO-E | DMF | 10 | 0.314 | 3.044 | 490 |
| Sample | DSC Max Peak (°C) | ΔH (J/g) |
|---|---|---|
| PAN-A | 324 | 5036 |
| PAN/LCGO-B | 323 | 4827 |
| PAN/LCGO-C | 325 | 4660 |
| PAN/LCGO-D | 327 | 4818 |
| PAN/LCGO-E | 329 | 4366 |
| Sample | PAN-A | PAN/LCGO-B | PAN/LCGO-C | PAN/LCGO-D | PAN/LCGO-E |
|---|---|---|---|---|---|
| Young’s Modulus (MPa) | 145.35 | 312.5 | 332.45 | 366.42 | 233.56 |
| Stress (MPa) | 19.60 | 22.90 | 31.20 | 41.10 | 16.5 |
| Strain (%) | 75.53 | 62.32 | 93.40 | 119.10 | 13.15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehrpouya, F.; Foroughi, J.; Naficy, S.; Razal, J.M.; Naebe, M. Nanostructured Electrospun Hybrid Graphene/Polyacrylonitrile Yarns. Nanomaterials 2017, 7, 293. https://doi.org/10.3390/nano7100293
Mehrpouya F, Foroughi J, Naficy S, Razal JM, Naebe M. Nanostructured Electrospun Hybrid Graphene/Polyacrylonitrile Yarns. Nanomaterials. 2017; 7(10):293. https://doi.org/10.3390/nano7100293
Chicago/Turabian StyleMehrpouya, Fahimeh, Javad Foroughi, Sina Naficy, Joselito M. Razal, and Minoo Naebe. 2017. "Nanostructured Electrospun Hybrid Graphene/Polyacrylonitrile Yarns" Nanomaterials 7, no. 10: 293. https://doi.org/10.3390/nano7100293
APA StyleMehrpouya, F., Foroughi, J., Naficy, S., Razal, J. M., & Naebe, M. (2017). Nanostructured Electrospun Hybrid Graphene/Polyacrylonitrile Yarns. Nanomaterials, 7(10), 293. https://doi.org/10.3390/nano7100293