Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents
Abstract
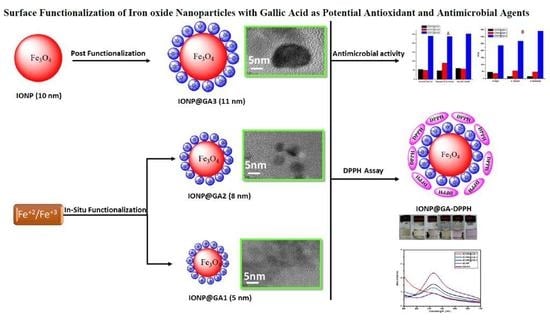
:1. Introduction
2. Results
2.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.2. Raman Spectra
2.3. X-ray Diffraction(XRD) Analysis
2.4. Morphological Characterization
2.5. Magnetic Properties
2.6. Energy Dispersive X-ray Spectroscopy (EDX) Analysis
2.7. Prediction Activity Spectra of Substances (PASS) of Biological Activity
2.8. Antioxidant Activity
2.9. Antimicrobial Activity
2.9.1. Antibacterial Activity
2.9.2. Antifungal Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of IONP
4.2.1. In Situ Functionalization
Synthesis of Organic IONP@GA1
Synthesis of Organic IONP@GA2
4.2.2. Post-Functionalization
Synthesis of IONP@GA3
4.2.3. Antioxidant Activity
4.2.4. Antimicrobial Activity
Determination of Antibacterial Activity
Determination of Antifungal Properties
- R1 = radius of the pathogen away from the antagonist.
- R2 = radius of the pathogen towards the antagonist.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patrick-Iwuanyanwu, K.; Onyeike, E.; Adhikari, A. Isolation, identification and characterization of gallic acid derivatives from leaves of tapinanthus bangwensis. J. Nat. Prod. 2014, 7, 14–19. [Google Scholar]
- Erica, S.; Daniel, A.; Silvana, A. Artificial nanoparticle antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. 235–253. [Google Scholar]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (bht): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Bharali, D.J.; Armstrong, D.; Bharali, D. Oxidative stress and nanotechnology. Methods Protoc. 2013, 1028. [Google Scholar]
- Sandhir, R.; Yadav, A.; Sunkaria, A.; Singhal, N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015, 89, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Esumi, K.; Takei, N.; Yoshimura, T. Antioxidant-potentiality of gold–chitosan nanocomposites. Colloids Surf. B. Biointerfaces 2003, 32, 117–123. [Google Scholar] [CrossRef]
- BarathManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.K.; Youn, H.-s.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of caenorhabditis elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Hikosaka, K.; Iitsuka, M.; Kanayama, A.; Toshima, N.; Miyamoto, Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic. Res. 2007, 41, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Moglianetti, M.; De Luca, E.; Pedone, D.; Marotta, R.; Catelani, T.; Sartori, B.; Amenitsch, H.; Retta, S.F.; Pompa, P.P. Platinum nanozymes recover cellular ros homeostasis in an oxidative stress-mediated disease model. Nanoscale 2016, 8, 3739–3752. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Saikia, J.; Samdarshi, S.; Konwar, B. Investigation of antioxidant property of iron oxide particlesby 1′-1′ diphenylpicryl-hydrazyle (dpph) method. J. Magn. Magn. Mater. 2009, 321, 3621–3623. [Google Scholar] [CrossRef]
- Toth, I.Y.; Szekeres, M.; Turcu, R.; Saringer, S.; Illes, E.; Nesztor, D.; Tombacz, E. Mechanism of in situ surface polymerization of gallic acid in an environmental-inspired preparation of carboxylated core-shell magnetite nanoparticles. Langmuir 2014, 30, 15451–15461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekeres, M.; Toth, I.Y.; Illes, E.; Hajdu, A.; Zupko, I.; Farkas, K.; Oszlanczi, G.; Tiszlavicz, L.; Tombacz, E. Chemical and colloidal stability of carboxylated core-shell magnetite nanoparticles designed for biomedical applications. Int. J. Mol. Sci. 2013, 14, 14550–14574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, J.P.; Paul, S.; Konwar, B.K.; Samdarshi, S.K. Nickel oxide nanoparticles: A novel antioxidant. Colloids Surf. B. Biointerfaces 2010, 78, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor-κb activation in h9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, T.; Choi, I.-Y.; Soh, M.; Kim, D.; Kim, Y.-J.; Jang, H.; Yang, H.-S.; Kim, J.Y.; Park, H.-K.; et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem., Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Dargusch, R.; Raitano, J.; Chan, S.-W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006, 342, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Wang, X.; Zhu, J.; Yang, Y.; Tian, J.; Cui, W.; Ge, C.; Li, Y.; Pan, Y.; et al. Facile synthesis of magnetic core-shell nanocomposites for mri and ct bimodal imaging. J. Mater. Chem. B 2015, 3, 6905–6910. [Google Scholar] [CrossRef]
- Tudisco, C.; Cambria, M.T.; Sinatra, F.; Bertani, F.; Alba, A.; Giuffrida, A.E.; Saccone, S.; Fantechi, E.; Innocenti, C.; Sangregorio, C.; et al. Multifunctional magnetic nanoparticles for enhanced intracellular drug transport. J. Mater. Chem. B 2015, 3, 4134–4145. [Google Scholar] [CrossRef]
- Majeed, M.I.; Lu, Q.; Yan, W.; Li, Z.; Hussain, I.; Tahir, M.N.; Tremel, W.; Tan, B. Highly water-soluble magnetic iron oxide (fe3o4) nanoparticles for drug delivery: Enhanced in vitro therapeutic efficacy of doxorubicin and mion conjugates. J. Mater. Chem. B 2013, 1, 2874–2884. [Google Scholar] [CrossRef]
- Dorniani, D.; Hussein, M.Z.B.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of fe3o4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Pankhurst, Q.A.; Thanh, N.T. High performance multi-core iron oxide nanoparticles for magnetic hyperthermia: Microwave synthesis, and the role of core-to-core interactions. Nanoscale 2015, 7, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Chorny, M.; Hood, E.; Levy, R.J.; Muzykantov, V.R. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J. Control. Release 2010, 146, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K. Anti-oxidant activities of biopolymeric nanoparticles: Boon or bane! J. Pharm. Res. 2014, 8, 871–876. [Google Scholar]
- Samuel, E.L.G.; Duong, M.T.; Bitner, B.R.; Marcano, D.C.; Tour, J.M.; Kent, T.A. Hydrophilic carbon clusters as therapeutic, high-capacity antioxidants. Trends Biotechnol. 2014, 32, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial nanoparticle antioxidants. ACS Symp. Ser. 2011, 1083, 235–253. [Google Scholar]
- Cîrcu, M.; Nan, A.; Borodi, G.; Liebscher, J.; Turcu, R. Refinement of magnetite nanoparticles by coating with organic stabilizers. Nanomaterials 2016, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodriguez, L.; Lafontaine, M.M.; Castro, C.; Mendez-Vega, J.; Latorre-Esteves, M.; Juan, E.J.; Mora, E.; Torres-Lugo, M.; Rinaldi, C. Synthesis, stability, cellular uptake, and blood circulation time of carboxymethyl-inulin coated magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Nasirimoghaddam, S.; Zeinali, S.; Sabbaghi, S. Chitosan coated magnetic nanoparticles as nano-adsorbent for efficient removal of mercury contents from industrial aqueous and oily samples. J. Ind. Eng. Chem. 2015, 27, 79–87. [Google Scholar] [CrossRef]
- Tancredi, P.; Botasini, S.; Moscoso-Londoño, O.; Méndez, E.; Socolovsky, L. Polymer-assisted size control of water-dispersible iron oxide nanoparticles in range between 15 and 100 nm. Colloids Surf. Physicochem. Eng. Asp. 2015, 464, 46–51. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Gogoi, B.; Buragohain, A.K.; Deb, P. Fe2o3/c nanocomposites having distinctive antioxidant activity and hemolysis prevention efficiency. Mater. Sci. Eng., C 2014, 42, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Sodipo, B.K.; Abdul Aziz, A. Non-seeded synthesis and characterization of superparamagnetic iron oxide nanoparticles incorporated into silica nanoparticles via ultrasound. Ultrason. Sonochem. 2015, 23, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Avti, P.; Pouliot, P.; Maafi, F.; Tardif, J.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Fabricating water dispersible superparamagnetic iron oxide nanoparticles for biomedical applications through ligand exchange and direct conjugation. Nanomaterials 2016, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Singh, R.M.; Mathur, S.C.; Singh, G.N. Quantification of gallic acid by hplc and antioxidant activity of amla fruits. J. Pharm. Res. 2007, 6, 161–162. [Google Scholar]
- Li, L.; Ng, T.B.; Gao, W.; Li, W.; Fu, M.; Niu, S.M.; Zhao, L.; Chen, R.R.; Liu, F. Antioxidant activity of gallic acid from rose flowers in senescence accelerated mice. Life Sci. 2005, 77, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Stanely Mainzen Prince, P.; Kumar, M.R.; Selvakumari, C.J. Effects of gallic acid on brain lipid peroxide and lipid metabolism in streptozotocin-induced diabetic wistar rats. J. Biochem. Mol. Toxicol. 2010, 25, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Ejakpovi, I.I.; Oyeleye, S.I. Antioxidant and antidiabetic effects of gallic and protocatechuic acids: A structure-function perspective. Comp. Clin Pathol 2015, 24, 1579–1585. [Google Scholar] [CrossRef]
- Locatelli, C.; Filippin-Monteiro, F.B.; Centa, A.; Creczinsky-Pasa, T.B. Antioxidant, antitumoral and anti-inflammatory activities of gallic acid. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Nova Science Publishers: New York, NY, USA, 2013. [Google Scholar]
- Seo, D.-J.; Lee, H.-B.; Kim, I.-S.; Kim, K.-Y.; Park, R.-D.; Jung, W.-J. Antifungal activity of gallic acid purified from terminalia nigrovenulosa bark against fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Shiraishi, Y.; Hisamatsu, F.; Miyamoto, A.; Kajita, M. Antioxidant for Cosmetic, External Application Medicine, and Food and Drink. Patent JP2008156440A, 2008. [Google Scholar]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical sio2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lacramioara, L.; Diaconu, A.; Butnaru, M.; Verestiuc, L. Biocompatible spions with superoxid dismutase/catalase immobilized for cardiovascular applications. In 3rd International Conference on Nanotechnologies and Biomedical Engineering; Sontea, V., Tiginyanu, I., Eds.; Springer: Singapore, 2016; Volume 55, pp. 323–326. [Google Scholar]
- Li, Y.; Lin, R.; Wang, L.; Huang, J.; Wu, H.; Cheng, G.; Zhou, Z.; MacDonald, T.; Yang, L.; Mao, H. Peg-b-age polymer coated magnetic nanoparticle probes with facile functionalization and anti-fouling properties for reducing non-specific uptake and improving biomarker targeting. J. Mater. Chem. B 2015, 3, 3591–3603. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Illés, E.; Janko, C.; Farkas, K.; Tóth, I.Y.; Nesztor, D.; Zupkó, I.; Földesi, I.; Alexiou, C.; Tombácz, E. Hemocompatibility and biomedical potential of poly(gallic acid) coated iron oxide nanoparticles for theranostic use. J. Nanomed. Nanotechnol. 2015, 6. [Google Scholar]
- De Faria, D.; Venâncio Silva, S.; De Oliveira, M. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (fe3o4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Francisco, M.; Teresa, C.; María, C.; Ramón, P.; Rolando, R.; Pedro, F.; José María, S.; Eduardo, E.; Carmen, M. Synthesis and characterization of monodisperse magnetite hollow microspheres. Soft Nanosci. Lett. 2011, 1, 25–32. [Google Scholar]
- Iyengar, S.J.; Joy, M.; Ghosh, C.K.; Dey, S.; Kotnala, R.K.; Ghosh, S. Magnetic, x-ray and mossbauer studies on magnetite/maghemite core-shell nanostructures fabricated through an aqueous route. RSC Adv. 2014, 4, 64919–64929. [Google Scholar] [CrossRef]
- Krekel, C. The chemistry of historical iron gall inks: Understanding the chemistry of writing inks used to prepare historical documents. Int. J. Forensic Doc. Exam. 1999, 5, 54–58. [Google Scholar]
- Wang, X.; Tilley, R.D.; Watkins, J.J. Simple ligand exchange reactions enabling excellent dispersibility and stability of magnetic nanoparticles in polar organic, aromatic, and protic solvents. Langmuir 2014, 30, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ma, H.-L.; Qi, X.-R.; Maitani, Y.; Nagai, T. Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. Int. J. Pharm. 2007, 333, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Dorniani, D.; Bin, H.M.Z.; Kura, A.U.; Fakurazi, S.; Hussein-Al-Ali, S.H.; Shaari, A.H.; Ahmad, Z. In vitro sustained release study of gallic acid coated with magnetite-peg and magnetite-pva for drug delivery system. Sci. World J. 2014, 1–11. [Google Scholar]
- Kareem, H.S.; Ariffin, A.; Nordin, N.; Heidelberg, T.; Abdul-Aziz, A.; Kong, K.W.; Yehye, W.A. Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3, 4, 5-trimethoxy benzyl moiety. Eur. J. Med. Chem. 2015, 103, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.V.; Filimonov, D.A.; Ihlenfeldt, W.-D.; Gloriozova, T.A.; Lagunin, A.A.; Borodina, Y.V.; Stepanchikova, A.V.; Nicklaus, M.C. Pass biological activity spectrum predictions in the enhanced open nci database browser. J. Chem. Inf. Comput. Sci. 2003, 43, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Yehye, W.A. Pass-predicted vitex negundo activity: Antioxidant and antiproliferative properties on human hepatoma cells-an in vitro study. BMC Complement. Altern. Med. 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stepanchikova, A.; Lagunin, A.; Filimonov, D.; Poroikov, V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Anzali, S.; Barnickel, G.; Cezanne, B.; Krug, M.; Filimonov, D.; Poroikov, V. Discriminating between drugs and nondrugs by prediction of activity spectra for substances (pass). J. Med. Chem. 2001, 44, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Kamalidehghan, B.; Ahmadipour, F.; Yehye, W.A. Pass-predicted hepatoprotective activity of caesalpinia sappan in thioacetamide-induced liver fibrosis in rats. Sci. World J. 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- PharmaExpert. Available online: http://www.pharmaexpert.ru/passonline/ (accessed on 4 April 2015).
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Sarangi, B.K.; Satpute, D.; Pandey, R.A. Synthesis and characterization of silver nanoparticles using cynodon dactylon leaves and assessment of their antibacterial activity. Bioprocess. Biosyst. Eng. 2013, 36, 999–1004. [Google Scholar] [CrossRef] [PubMed]











| Sample | Ms (emu g−1) |
|---|---|
| IONP | 64.19 |
| IONP@GA1 | 43.90 |
| IONP@GA2 | 60.26 |
| IONP@GA3 | 53.43 |
| Sample | A | B | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | O | C | N | Fe | O | C | N | |
| IONP | 69.6 | 39.4 | - | - | 77.5 | 21.2 | 1.3 | - |
| IONP@GA1 | 62 | 30.2 | 7 | 0.6 | 62.6 | 29.5 | 7.2 | 0.8 |
| IONP@GA2 | 65.6 | 29.1 | 4.8 | 0.4 | 63.7 | 29.7 | 6 | 0.6 |
| IONP@GA3 | 58.7 | 30 | 10.8 | - | 61.5 | 26.4 | 11.7 | 0.3 |
| Biological Activity | Pa a | Pi b |
|---|---|---|
| Antioxidant | 0.529 | 0.005 |
| Free Radical Scavenger | 0.579 | 0.007 |
| Lipid Peroxidase Inhibitor | 0.554 | 0.012 |
| Anti-inflammatory | 0.560 | 0.041 |
| Antibacterial | 0.420 | 0.026 |
| Antibacterial, ophthalmic | 0.255 | 0.005 |
| Antifungal | 0.255 | 0.050 |
| Antifungal (Pneumocystis) | 0.109 | 0.003 |
| IC50 a Values (mg/mL) ± S.E.M b and Max. Inhibition % | |||
|---|---|---|---|
| Sample | IC50 mg/mL | % Inhibition | |
| IONP@GA3 | 5 mg | 1.00 ± 0.003 | 78 |
| IONP@GA2 | 5 mg | 2.2 ± 0.002 | 59 |
| IONP@GA1 | 5 mg | 2.7 ± 0.003 | 61 |
| IONP | 5 mg | 4.7 ± 0.002 | 50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.T.; A Yehya, W.; Saad, O.; Simarani, K.; Chowdhury, Z.; A. Alhadi, A.; Al-Ani, L.A. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. https://doi.org/10.3390/nano7100306
Shah ST, A Yehya W, Saad O, Simarani K, Chowdhury Z, A. Alhadi A, Al-Ani LA. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials. 2017; 7(10):306. https://doi.org/10.3390/nano7100306
Chicago/Turabian StyleShah, Syed Tawab, Wageeh A Yehya, Omer Saad, Khanom Simarani, Zaira Chowdhury, Abeer A. Alhadi, and Lina A. Al-Ani. 2017. "Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents" Nanomaterials 7, no. 10: 306. https://doi.org/10.3390/nano7100306
APA StyleShah, S. T., A Yehya, W., Saad, O., Simarani, K., Chowdhury, Z., A. Alhadi, A., & Al-Ani, L. A. (2017). Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials, 7(10), 306. https://doi.org/10.3390/nano7100306