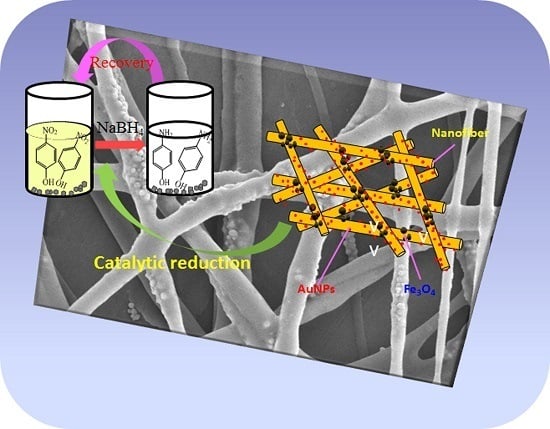
Hierarchical AuNPs-Loaded Fe3O4/Polymers Nanocomposites Constructed by Electrospinning with Enhanced and Magnetically Recyclable Catalytic Capacities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospun Composites
2.3. Catalytic Performance Test
2.4. Characterization
3. Results and Discussion
3.1. Characterization of Nanocomposites
3.2. Catalytic Reduction Performances
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bond, G.C.; Sermon, P.A. Gold catalysts for olefin hydrogenation. Gold Bull. 1973, 6, 102–105. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Hutchings, G.J. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Villaverde, G.; Corma, A.; Iglesias, M.; Sanchez, F. Heterogenized gold complexes: Recoverable catalysts for multicomponent reactions of aldehydes, terminal alkynes, and amines. ACS Catal. 2016, 2, 399–406. [Google Scholar] [CrossRef]
- Sanchez, A.; Abbet, S.; Heiz, U.; Schneider, W.D.; Hakkinen, H. When gold is not noble: Nanoscale gold catalysts. J. Phys. Chem. A 1999, 103, 9573–9678. [Google Scholar] [CrossRef]
- Hernández, J.; Sollagullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. Electrochemistry of shape-controlled catalysts: Oxygen reduction reaction on cubic gold nanoparticles. J. Phys. Chem. C 2015, 111, 14078–14083. [Google Scholar] [CrossRef]
- Kundu, M.K.; Bhowmik, T.; Barman, S. Gold aerogel supported on graphitic carbon nitride: An efficient electrocatalyst for oxygen reduction reaction and hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 23120–23135. [Google Scholar] [CrossRef]
- Zheng, G.; Polavarapu, L.; Lizmarzán, L.M.; Pastorizasantos, I.; Perezjuste, J. Gold nanoparticle-loaded filter paper: A recyclable dip-catalyst for real-time reaction monitoring by surface enhanced Raman scattering. Chem. Commun. 2015, 51, 4572–4575. [Google Scholar] [CrossRef] [PubMed]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Gold-indium modified TiO2, nanocatalysts for photocatalytic CO2, reduction with H2, as reductant in a monolith photoreactor. Appl. Surf. Sci. 2015, 338, 1–14. [Google Scholar] [CrossRef]
- Sandoval, A.; Zanella, R.; Klimova, T.E. Titania nanotubes decorated with anatase nanocrystals as support for active and stable gold catalysts for CO oxidation. Catal. Today 2017, 282, 140–150. [Google Scholar] [CrossRef]
- Chen, S.; Luo, L.; Jiang, Z.; Huang, W. Size-dependent reaction pathways of low-temperature CO oxidation on Au/CeO2 catalysts. ACS Catal. 2015, 5, 75–78. [Google Scholar] [CrossRef]
- Sinha, A.K.; Seelan, S.; Tsubota, S.; Haruta, M. A three-dimensional mesoporous titanosilicate support for gold nanoparticles: Vapor-phase epoxidation of propene with high conversion. Angew. Chem. Int. Ed. 2004, 43, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.; Bravo-Suárez, J.J.; Daté, M.; Tsubota, S.; Haruta, M. Trimethylamine as a gas-phase promoter: Highly efficient epoxidation of propylene over supported gold catalysts. Angew. Chem. Int. Ed. 2006, 45, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Wang, Y.N.; Zhang, J.H.; Wang, D.Y.; Ma, W.H. Better performance for gas-phase epoxidation of propylene using H2 and O2 at lower temperature over Au/TS-1 catalyst. Catal. Commun. 2017, 90, 87–90. [Google Scholar] [CrossRef]
- Chang, M.W.; Sheu, W.S. Water-gas-shift reaction on reduced gold-substituted Ce1-xO2 (111) surfaces: The role of Au charge. Phys. Chem. Chem. Phys. 2017, 19, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.X.; Mahr, C.; Murshed, M.M.; Zielasek, V.; Rosenauer, A.; Guesing, T.; Bäumer, M.; Wittstock, A. A versatile sol-gel coating for mixed oxides on nanoporous gold and their application in the water gas shift reaction. Catal. Sci. Technol. 2016, 6, 5311–5319. [Google Scholar] [CrossRef]
- Yao, S.Y.; Zhang, X.; Zhou, W.; Gao, R.; Xu, W.Q.; Ye, Y.F.; Lin, L.L.; Wen, X.D.; Liu, P.P.; Chen, B.B.; et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 2017, 357, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Kapkowski, M.; Bartczak, P.; Korzec, M.; Sitko, R.; Szade, J.; Balin, K.; Lelatko, J.; Polanski, J. SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation in the liquid phase. J. Catal. 2014, 319, 110–118. [Google Scholar] [CrossRef]
- Dong, W.; Reichenberger, S.; Chu, S.; Weide, P.; Ruland, H.; Barcikowski, S.; Wagener, P.; Muhler, M. The effect of the Au loading on the liquid-phase aerobic oxidation of ethanol over Au/TiO2, catalysts prepared by pulsed laser ablation. J. Catal. 2015, 330, 497–506. [Google Scholar] [CrossRef]
- Wang, T.; Yuan, X.; Li, S.; Gong, J. CeO2-modified Au@SBA-15 nanocatalysts for liquid-phase selective oxidation of benzyl alcohol. Nanoscale 2015, 7, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Heeskens, D.; Aghaei, P.; Kaluza, S.; Strunk, J.; Muhler, M. Selective oxidation of ethanol in the liquid phase over Au/TiO2. Phys. Status Solidi B 2013, 250, 1107–1118. [Google Scholar] [CrossRef]
- Evangelista, V.; Acosta, B.; Miridonov, S.; Smolentseva, E.; Fuentes, S.; Simakov, A. Highly active Au-CeO2@ZrO2, yolk-shell nanoreactors for the reduction of 4-nitrophenol to 4-aminophenol. Appl. Catal. B Environ. 2015, 166, 518–528. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Zhang, J.; Yan, X.; Chen, Z. Fe3O4 and Au nanoparticles dispersed on the graphene support as a highly active catalyst toward the reduction of 4-nitrophenol. Phys. Chem. Chem. Phys. 2016, 18, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.D.; Wang, Y.X.; Xi, S.F.; Li, Y.X.; Li, Z.J.; Ren, X.H.; Gu, Z.G. A hexagonal covalent porphyrin framework as an efficient support for gold nanoparticles toward catalytic reduction of 4-Nitrophenol. Chem. Eur. J. 2016, 22, 17029–17036. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Yang, Y.X.; Ma, M.G.; Wang, X.M.; Zhen, D.X. Self-assembled gold nanoparticles coating for solid-phase microextraction of ultraviolet filters in environmental water. Chin. J. Anal. Chem. 2015, 43, 207–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.H.; Yang, X.; Huang, J.; Chen, W.; Lv, X.M.; Li, C.Y.; Li, C.Z. Au decorated Fe3O4@TiO2 magnetic composites with visible light-assisted enhanced catalytic reduction of 4-Nitrophenol. RSC Adv. 2015, 5, 50454–50461. [Google Scholar] [CrossRef]
- Lau, M.; Ziefuss, A.; Komossa, T.; Barcikowski, S. Inclusion of supported gold nanoparticles into their semiconductor support. Phys. Chem. Chem. Phys. 2015, 17, 29311–29318. [Google Scholar] [CrossRef] [PubMed]
- Tvauri, I.V.; Gergieva, B.E.; Magkoeva, V.D.; Grigorkina, G.S.; Bliev, A.P.; Ashkhotov, O.G.; Spzaev, V.A.; Fukutani, K.; Magkoev, T.T. Carbon monoxide oxidation on lithium fluoride supported gold nanoparticles: A significance of F-centers. Solid State Commun. 2015, 213–214, 42–45. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Guo, X.; Wu, S.; Wang, S. Porous alpha-Fe2O3 decorated by Au nanoparticles and their enhanced sensor performance. Nanotechnology 2010, 21. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Ma, X.; Yang, J.; Cai, W.; Zhu, G.; Liu, J. Preparation of Au nanoparticles decorated polyaniline nanotube and its catalytic oxidation to ascorbic acid. Chem. Res. Chin. Univ. 2016, 32, 1–7. [Google Scholar] [CrossRef]
- Chairam, S.; Konkamdee, W.; Parakhun, R. Starch-supported gold nanoparticles and their use in 4-nitrophenol reduction. J. Saudi. Chem. Soc. 2015. [Google Scholar] [CrossRef]
- Zhu, C.; Han, L.; Hu, P.; Dong, S. Loading of well-dispersed gold nanoparticles on two-dimensional graphene oxide/SiO composite nanosheets and their catalytic properties. Nanoscale 2012, 4, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-Aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Lu, W.; Wang, L.; Tian, J.; Sun, X. In situ green synthesis of Au nanostructures on graphene oxide and their application for catalytic reduction of 4-Nitrophenol. Catal. Sci. Technol. 2011, 1, 1142–1144. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.M.; Xie, D.S. Green synthesis of Pt-Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4-nitrophenol reduction. Appl. Catal. B Environ. 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Jin, C.; Han, J.; Chu, F.; Guo, R. Fe3O4@PANI hybrid shell as a multifunctional support for Au nanocatalysts with a rmarkably improved catalytic performance. Langmuir 2017, 33, 4520–4527. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Yarin, A.L. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol. 2015, 22, 310–317. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Lovett, M.L.; Kaplan, D.L.; Bhowmick, S. Role of electrospun fibre diameter and corresponding specific surface area (SSA) on cell attachment. J. Tissue Eng. Regen. Med. 2009, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, Y.; Miao, Y.E.; Weng, W.T.; Liu, T. Polydopamine-coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Wang, W.; Jiao, T.; Ma, K.; Zhang, Q.; Hong, W.; Qiu, H.; Zhou, J.; Zhang, L.; Peng, Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- He, D.; Hu, B.; Yao, Q.F.; Wang, K.; Yu, S.H. Large-scale synthesis of flexible free-standing SERS substrates with high sensitivity: Electrospun PVA nanofibers embedded with controlled alignment of silver nanoparticles. ACS Nano 2009, 3, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, J.; Dong, X.; Yu, W.; Liu, G. Fabrication of magnetic-fluorescent bifunctional flexible coaxial nanobelts by electrospinning using a modified coaxial spinneret. Chempluschem 2014, 79, 290–297. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Ma, K.; Jiao, T.; Xing, R.; Li, K.; Zhou, J.; Zhang, L. Preparation and dye removal capacities of porous silver nanoparticle-containing composite hydrogels via poly(acrylic acid) and silver ions. RSC Adv. 2016, 6, 110799–110807. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Li, X.; Zhang, M.Y.; Zhang, X.; Sun, Y.Y.; Liu, X. In situ assembly of well-dispersed Au nanoparticles on TiO2/ZnO nanofibers: A three-way synergistic heterostructure with enhanced photocatalytic activity. J. Hazard. Mater. 2012, 237, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhou, Y.; Huang, J.; Zhu, Y.H.; Zhu, J.R.; Yang, X.L.; Chen, W.; Yao, Y.F.; Qian, S.H.; Jiang, H.; et al. In-situ SERS monitoring of reaction catalyzed by multifunctional Fe3O4@TiO2@Ag-Au microspheres. Appl. Catal. B Environ. 2017, 205, 11–18. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, S.; Gao, W.; Jiang, P.B.; Li, R. Au/Fe3O4@TiO2, hollow nanospheres as efficient catalysts for the reduction of 4-nitrophenol and photocatalytic degradation of rhodamine B. React. Kinet. Mech. Catal. 2017, 121, 797–810. [Google Scholar] [CrossRef]
- Li, J.; Tan, L.; Wang, G.; Yang, M. Synthesis of double-shelled sea urchin-like yolk-shell Fe3O4/TiO2/Au microspheres and their catalytic applications. Nanotechnology 2015, 26, 095601. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.L.; Jiao, T.F.; Xing, R.R.; Chen, Y.; Zhou, J.X.; Zhang, L.X. Preparation of TiO2 nanoparticles modified electrospun nanocomposite membranes toward efficient dye degradation for wastewater treatment. J. Taiwan Inst. Chem. Eng. 2017, 78, 118–126. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, H.X.; Guo, W.C.; Li, R.F.; Tian, K.S.; Xu, Z.P.; Jia, Y.; Wu, Y.H.; Cao, L. In situ confined growth based on a self-templating reduction strategy of highly dispersed Ni nanoparticles in hierarchical yolk-shell Fe@SiO2 structures as efficient catalysts. Chem. Asian J. 2016, 11, 3534–4350. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.C.; Wang, Q.; Wang, G.; Yang, M.; Dong, W.J.; Yu, J. Facile hydrogen-bond-assisted polymerization and immobilization method to synthesize hierarchical Fe3O4@poly(4-vinylpyridine-co-divinylbenzene)@Au nanostructures and their catalytic applications. Chem. Asian J. 2013, 8, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.R.; Liu, K.; Jiao, T.F.; Zhang, N.; Ma, K.; Zhang, R.Y.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/ohotodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, K.; Jiao, T.; Xing, R.; Shen, G.; Yan, X. Water-insoluble photosensitizer nanocolloids stabilized by supramolecular interfacial assembly towards photodynamic therapy. Sci. Rep. 2017, 7, 42978. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Jiao, T.; Liu, Y.; Ma, K.; Zou, Q.; Ma, G.; Yan, X. Co-assembly of graphene oxide and albumin/photosensitizer nanohybrids towards enhanced photodynamic therapy. Polymers 2016, 8, 181. [Google Scholar] [CrossRef]
- Zhao, X.N.; Ma, K.; Jiao, T.F.; Xing, R.R.; Ma, X.L.; Hu, J.; Huang, H.; Zhang, L.; Yan, X. Fabrication of hierarchical layer-by-layer assembled diamond-based core-shell nanocomposites as highly efficient dye absorbents for wastewater treatment. Sci. Rep. 2017, 7, 44076. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xing, R.; Jiao, T.; Ma, K.; Chen, C.; Ma, G.; Yan, X. Carrier-free, chemo-photodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Li, X.Y.; Sun, G.; Zhang, G.L.; Liu, H.; Du, J.S.; Yao, S.T.; Bai, J.; Yang, Q.B. Fabrication of Au/PVP nanofiber composites by electrospinning. J. Appl. Polym. Sci. 2010, 105, 3618–3622. [Google Scholar] [CrossRef]
- Kundu, S.; Gill, R.S.; Saraf, R.F. Electrospinning of PAH nanofiber and deposition of Au NPs for nanodevice fabrication. J. Phys. Chem. C 2011, 115, 15845–15852. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Huan, Y.; Li, Z.; He, G.; Liu, M. Self-assembled supramolecular nanotube yarn. Adv. Mater. 2013, 25, 5875–5879. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, T.; Liu, M. Macroscopic chirality of supramolecular gels formed from achiral tris(ethyl cinnamate) benzene-1,3,5-tricarboxamides. Angew. Chem. Int. Ed. 2014, 53, 13424–13428. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Cai, Y.; Sun, Z.K.; Liu, J.; Liu, C.; Wei, J.; Li, W.; Liu, C.; Wang, Y.; Zhao, D.Y. Multifunctional mesoporous composite microspheres with well-designed nanostructure: A highly integrated catalyst system. J. Am. Chem. Soc. 2010, 132, 8466–8473. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Full- and white color circularly polarized luminescent quantum dots via supramolecular self-assembly. Angew. Chem. Int. Ed. 2017, 129, 12342–12346. [Google Scholar] [CrossRef]
- Garcia-Serrano, J.; Galindo, A.G.; Pal, U. Au-Al2O3 nanocomposites: XPS and FTIR spectroscopic studies. Sol. Energy Mater. Sol. Cell 2004, 82, 291–298. [Google Scholar] [CrossRef]










| No. | Catalyzer | Catalytic Performance ln(Ct/C0) min−1 | Preparation Method | Characteristics |
|---|---|---|---|---|
| 1 | Au@CPF-1 hybrid [24] | 0.303 | AuNPs synthesized on the activated CPF-1. | Complexed and costly preparation. |
| 2 | Starch-supported gold nanoparticles [32] | - | Mix HAuCl4 and MBS in DI water. | Weak reducibility of polysaccharides, weak catalytic activity, simple process, and environmentally friendly. |
| 3 | Graphene oxide/SiO2/AuNPs hybrid nanomaterials [33] | 1.04 | Graphene oxide/SiO2 via a sol–gel process, activated by SnCl2, mixed with HAuCl4. | Remarkable catalytic capacity, accompanying adsorption process, inconvenient preparation process. |
| 4 | TiO2/ZnO/AuNF nanofibers [48] | - | Calcined electrospinning nanofibers, SnCl2 activated, adding HAuCl4 solution. | Toxic solvent in preparation, unfriendly to environment. |
| 5 | Fe3O4@TiO2@Ag–Au microspheres [49] | 0.1148 | 3-Aminopropyltrimethoxysilane modified Fe3O4@TiO2 microspheres, Ag nanoparticles replacement, Ag–Au bimetallic nanostructures. | Complexed replacement of Au/Ag, weak catalytic activity. |
| 6 | Au/Fe3O4@hollow TiO2 nanoreactor [50] | 0.46 | AuNPs loaded on magnetic SiO2 nanospheres, Fe3O4 modified, covered with TiO2 shell. | Impacted catalytic capacity due to the coverage and isolation of the TiO2 shell. |
| 7 | Double-shelled sea urchin-like yolk-shell Fe3O4/TiO2/Au microspheres [51] | 1.84 | Synthesis of Fe3O4/SiO2/TiO2 core-shell microspheres by sol–gel process, SiO2 shell removed by acid post-treatment, AuNPs loaded. | Remarkable catalytic performance, complexed preparation, negative effect in acid post-treatment. |
| 8 | Present work | 0.441 | AuNPs-loaded, magnetically Fe3O4 support by electrospinning. | Eco-friendly prepared process, high stability, and good catalytic performance. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Jiao, T.; Xing, R.; Chen, Y.; Guo, W.; Zhou, J.; Zhang, L.; Peng, Q. Hierarchical AuNPs-Loaded Fe3O4/Polymers Nanocomposites Constructed by Electrospinning with Enhanced and Magnetically Recyclable Catalytic Capacities. Nanomaterials 2017, 7, 317. https://doi.org/10.3390/nano7100317
Guo R, Jiao T, Xing R, Chen Y, Guo W, Zhou J, Zhang L, Peng Q. Hierarchical AuNPs-Loaded Fe3O4/Polymers Nanocomposites Constructed by Electrospinning with Enhanced and Magnetically Recyclable Catalytic Capacities. Nanomaterials. 2017; 7(10):317. https://doi.org/10.3390/nano7100317
Chicago/Turabian StyleGuo, Rong, Tifeng Jiao, Ruirui Xing, Yan Chen, Wanchun Guo, Jingxin Zhou, Lexin Zhang, and Qiuming Peng. 2017. "Hierarchical AuNPs-Loaded Fe3O4/Polymers Nanocomposites Constructed by Electrospinning with Enhanced and Magnetically Recyclable Catalytic Capacities" Nanomaterials 7, no. 10: 317. https://doi.org/10.3390/nano7100317
APA StyleGuo, R., Jiao, T., Xing, R., Chen, Y., Guo, W., Zhou, J., Zhang, L., & Peng, Q. (2017). Hierarchical AuNPs-Loaded Fe3O4/Polymers Nanocomposites Constructed by Electrospinning with Enhanced and Magnetically Recyclable Catalytic Capacities. Nanomaterials, 7(10), 317. https://doi.org/10.3390/nano7100317