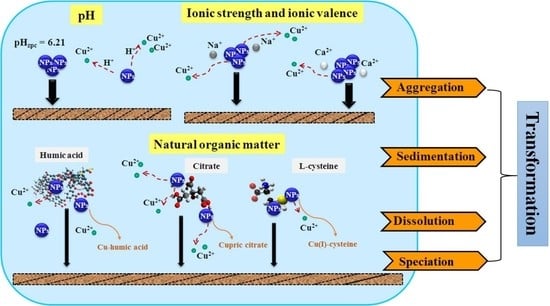
Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter
Abstract
:1. Introduction
2. Results
2.1. Properties of CuO NPs
2.2. Effect of pH on the Agglomeration of CuO NPs
2.3. Effect of Ionic Strength and Ionic Valence on the Agglomeration of CuO NPs
2.4. Effect of NOM on the Agglomeration of CuO NPs
2.5. Sedimentation of CuO NPs Exposed to Varying pH, Electrolyte, and NOM
2.6. Dissolution of CuO NPs Exposed to Varying pH, Electrolytes, and NOM
2.7. Speciation of CuO NPs in the Presence with NOM
3. Discussion
4. Materials and Methods
4.1. Characterization of CuO NPs
4.2. Solution Chemistry
4.3. Aggregation and Zeta Potential Measurements
4.4. Analysis of the Interaction between Particles by DLVO Theory
4.5. Sedimentation Study
4.6. Dissolution Measurements
4.7. XANES Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lead, J.R.; Smith, E. Environmental and Human Health Impacts of Nanotechnology; Wiley: Chichester, UK, 2009. [Google Scholar]
- Cioffi, N.; Ditaranto, N.; Torsi, L.; Picca, R.A.; Sabbatini, L.; Valentini, A.; Novello, L.; Tantillo, G.; Bleve-Zacheo, T.; Zambonin, P.G. Analytical characterization of bioactive fluoropolymer ultra-thin coatings modified by copper nanoparticles. Anal. Bioanal. Chem. 2005, 381, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Saison, C.; Perreault, F.; Daigle, J.-C.; Fortin, C.; Claverie, J.; Morin, M.; Popovic, R. Effect of core–shell copper oxide nanoparticles on cell culture morphology and photosynthesis (photosystem II energy distribution) in the green alga, Chlamydomonas reinhardtii. Aquat. Toxicol. 2010, 96, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Shi, W.H.; Zhu, J.X.; Kharistal, D.J.; Zhao, W.Y.; Lalia, B.S.; Hng, H.H.; Yan, Q.Y. High-power and high-energy-density flexible pseudocapacitor electrodes made from porous CuO nanobelts and single-walled carbon nanotubes. ACS Nano 2011, 5, 2013–2019. [Google Scholar] [CrossRef]
- Keller, A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Handy, R.D.; von der Kammer, F.; Lead, J.R.; Hassellov, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peng, B.; Hernandez-Viezcas, J.A.; Rico, C.; Sun, Y.; Peralta-Videa, J.R.; Tang, X.; Niu, G.; Jin, L.; Varela-Ramirez, A.; et al. Stress response and tolerance of zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano 2012, 6, 9615–9622. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, X.; Hammer, B.K.; Du, S.; Chen, Y. Nanoparticles inhibit DNA replication by binding to DNA: Modeling and experimental validation. ACS Nano 2013, 7, 9664–9674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Zhang, X.; Zhao, Z.; Liu, H.; George, R.; Wilson-Rawls, J.; Chang, Y.; Chen, Y. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 2011, 83, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangon, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125–1139. [Google Scholar] [CrossRef]
- Ivask, A.; Scheckel, K.G.; Kapruwan, P.; Stone, V.; Yin, H.; Voelcker, N.H.; Lombi, E. Complete transformation of ZnO and CuO nanoparticles in culture medium and lymphocyte cells during toxicity testing. Nanotoxicology 2017, 11, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chung, H.; Kim, S.; Lee, I. The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Pollut. 2013, 224, 1668. [Google Scholar] [CrossRef]
- Shaw, A.K.; Hossain, Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 2013, 93, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.M. Water Chemistry; McGraw-Hill: Boston, MA, USA, 2002. [Google Scholar]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2011, 28, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.T.; Livi, K.; Arai, Y. Effects of pH and phosphate on CeO2 nanoparticle dissolution. Chemosphere 2015, 119, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef]
- Zhou, D.; Keller, A.A. Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res. 2010, 44, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of suwannee river humic acid. Sci. Total Environ. 2014, 468, 195–201. [Google Scholar]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.M.; Grassian, V.H. Dissolution of ZnO nanoparticles at crcumneutral pH: A study of size effects in the presence and absence of citric acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Chen, K.L.; Mylon, S.E.; Elimelech, M. Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ. Sci. Technol. 2006, 40, 1516–1523. [Google Scholar] [CrossRef]
- Giasuddin, A.B.; Kanel, S.R.; Choi, H. Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ. Sci. Technol. 2007, 41, 2022–2027. [Google Scholar] [CrossRef]
- Sánchez-Cortés, S.; Francioso, O.; Ciavatta, C.; Garcĺa-Ramos, J.V.; Gessa, C. pH-dependent adsorption of fractionated peat humic substances on different silver colloids studied by surface-enhanced raman spectroscopy. J. Colloid Interface Sci. 1998, 198, 308–318. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Keller, A.A.; Wang, T.; Li, F. The effect of humic acid on the aggregation of titanium dioxide nanoparticles under different pH and ionic strengths. Sci. Total Environ. 2014, 487, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Rocchicciolideltcheff, C.; Franck, R.; Cabuil, V.; Massart, R. Surfacted ferrofluids—Interactions at the surfactant-magnetic iron-oxide interface. J. Chem. Res. 1987, 5, 126–127. [Google Scholar]
- Arancon, R.A.D.; Lin, S.H.; Chen, G.; Lin, C.S.K.; Lai, J.; Xu, G.; Luque, R. Nanoparticle tracking analysis of gold nanomaterials stabilized by various capping agents. RSC Adv. 2014, 4, 17114–17119. [Google Scholar] [CrossRef]
- Lu, C.-H.; Wang, Y.-W.; Ye, S.-L.; Chen, G.-N.; Yang, H.-H. Ultrasensitive detection of Cu2+ with the naked eye and application in immunoassays. NPG Asia Mater. 2012, 4, e10. [Google Scholar] [CrossRef]
- Lim, I.I.S.; Mott, D.; Ip, W.; Njoki, P.N.; Pan, Y.; Zhou, S.; Zhong, C.J. Interparticle interactions in glutathione mediated assembly of gold nanoparticles. Langmuir 2008, 24, 8857–8863. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, P.; Joseph, S.S.; Thomas, K.G. Selective detection of cysteine and glutathione using gold nanorods. J. Am. Chem. Soc. 2005, 127, 6516–6517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kou, X.; Yang, Z.; Shi, Q.; Stucky, G.D.; Sun, L.; Wang, J.; Yan, C. Nanonecklaces assembled from gold rods, spheres, and bipyramids. Chem. Commun. 2007, 1816–1818. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Humblot, V.; Méthivier, C.; Pradier, C.M. Glutathione adsorption from UHV to the liquid phase at various pHon gold and subsequent modification of protein interaction. Surf. Interface Anal. 2008, 40, 395–399. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Lu, C.H.; Willner, I. Cysteine-mediated aggregation of Au nanoparticles: The development of a H2O2 sensor and oxidase-based biosensors. ACS Nano 2013, 7, 7278–7286. [Google Scholar] [CrossRef] [PubMed]
- Dokken, K.M.; Parsons, J.G.; McClure, J.; Gardea-Torresdey, J.L. Synthesis and structural analysis of copper (II) cysteine complexes. Inorg. Chim. Acta 2009, 362, 395–401. [Google Scholar] [CrossRef]
- Koneswaran, M.; Narayanaswamy, R. l-cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sens. Actuator B Chem. 2009, 139, 104–109. [Google Scholar] [CrossRef]
- Yang, X.Y.; Gondikas, A.P.; Marinakos, S.M.; Auffan, M.; Liu, J.; Hsu-Kim, H.; Meyer, J.N. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 1119–1127. [Google Scholar]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research strategies for safety evaluation of nanomaterials, part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, C.; Liu, Q.; Sun, L.; Luo, Y.; Shi, J. Fate and transformation of CuO nanoparticles in the soil–rice system during the life cycle of rice plants. Environ. Sci. Technol. 2017, 51, 4907–4917. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, L.Y.; Han, J.T.; Zhang, J.Y.; Li, Z.Y.; Qian, D.J. Preparation and study of polyacryamide-stabilized silver nanoparticles through a one-pot process. J. Phys. Chem. B 2006, 110, 11224–11231. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lenhart, J.J.; Walker, H.W. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef] [PubMed]
- Mendham, J.; Denney, R.C.; Barnes, J.D.; Thomas, M.J.K.; Denney, R.C.; Thomas, M.J.K. Vogel’s Quantitative Chemical Analysis, 6th ed.; Prentice Hall: New York, NY, USA, 2000. [Google Scholar]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Cytotoxic origin of copper(II) oxide nanoparticles: Comparative studies with micron-sized particles, leachate, and metal salts. ACS Nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, D.; Hwang, G.; Lee, B.; Eom, I.; Kim, P.J.; Tong, M.; Kim, H. Aggregation and dissolution of ZnO nanoparticles synthesized by different methods: Influence of ionic strength and humic acid. Colloids Surf. A 2014, 451, 7–15. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Zhao, J.; Xing, B. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environ. Sci. Technol. 2011, 45, 6032–6040. [Google Scholar]
- Rigo, A.; Corazza, A.; Luisa di Paolo, M.; Rossetto, M.; Ugolini, R.; Scarpa, M. Interaction of copper with cysteine: Stability of cuprous complexes and catalytic role of cupric ions in anaerobic thiol oxidation. J. Inorg. Biochem. 2004, 98, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.J.; Zhang, X.Y.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Zinc oxide-engineered nanoparticles: Dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, H.; Fang, H.; Xu, C.; Huang, H.; Wang, Y.; Sun, L.; Yuan, X.; Chen, Y.; Shi, J. Natural organic matter-induced alleviation of the phytotoxicity to rice (Oryza sativa L.) caused by copper oxide nanoparticles. Environ. Toxicol. Chem. 2015, 34, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Peng, C.; Yang, Y.; Yang, J.; Zhang, H.; Yuan, X.; Chen, Y.; Hu, T. Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology 2014, 8, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Elimelech, M. Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 2006, 22, 10994–11001. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Fujita, K.; Horie, M.; Suzuki, M.; Nakamura, A.; Endoh, S.; Yoshida, Y.; Iwahashi, H.; Takahashi, K.; Kinugasa, S. Dispersion characteristics of various metal oxide secondary nanoparticles in culture medium for in vitro toxicology assessment. Toxicol. In Vitro 2010, 24, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Duan, D.; Xu, C.; Chen, Y.; Sun, L.; Zhang, H.; Yuan, X.; Zheng, L.; Yang, Y.; Yang, J.; et al. Translocation and biotransformation of CuO nanoparticles in rice (Oryza sativa L.) plants. Environ. Pollut. 2015, 197, 99–107. [Google Scholar] [CrossRef] [PubMed]







| Environmental Factors | pH | Ionic Strength (mM) | |||||||
| NaCl | CaCl2 | ||||||||
| 3 | 5 | 6 | 7 | 9 | 1 | 10 | 100 | 100 | |
| k (h−1) | 0.1180 | 0.2529 | 0.3054 | 0.2631 | 0.1415 | 0.1975 | 0.2631 | 0.4174 | 0.4260 |
| R2 | 0.9840 | 0.9509 | 0.9803 | 0.9932 | 0.9689 | 0.9888 | 0.9932 | 0.9930 | 0.9942 |
| NOM | Humic Acid Concentration (mg/L) | Citric Acid Concentration (mg/L) | l-cysteine Concentration (mg/L) | ||||||
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | |
| k (h−1) | 0.1944 | 0.1429 | 0.0266 | 0.0713 | 0.0663 | 0.2188 | 0.1510 | 0.0842 | 0.1288 |
| R2 | 0.9621 | 0.9444 | 0.7620 | 0.9209 | 0.9195 | 0.9621 | 0.9364 | 0.9595 | 0.8495 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials 2017, 7, 326. https://doi.org/10.3390/nano7100326
Peng C, Shen C, Zheng S, Yang W, Hu H, Liu J, Shi J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials. 2017; 7(10):326. https://doi.org/10.3390/nano7100326
Chicago/Turabian StylePeng, Cheng, Chensi Shen, Siyuan Zheng, Weiling Yang, Hang Hu, Jianshe Liu, and Jiyan Shi. 2017. "Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter" Nanomaterials 7, no. 10: 326. https://doi.org/10.3390/nano7100326
APA StylePeng, C., Shen, C., Zheng, S., Yang, W., Hu, H., Liu, J., & Shi, J. (2017). Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials, 7(10), 326. https://doi.org/10.3390/nano7100326