Unraveling the Origin of Magnetism in Mesoporous Cu-Doped SnO2 Magnetic Semiconductors
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis of Mesoporous Cu-Doped SnO2 Powders
2.3. Characterization
3. Results and Discussion
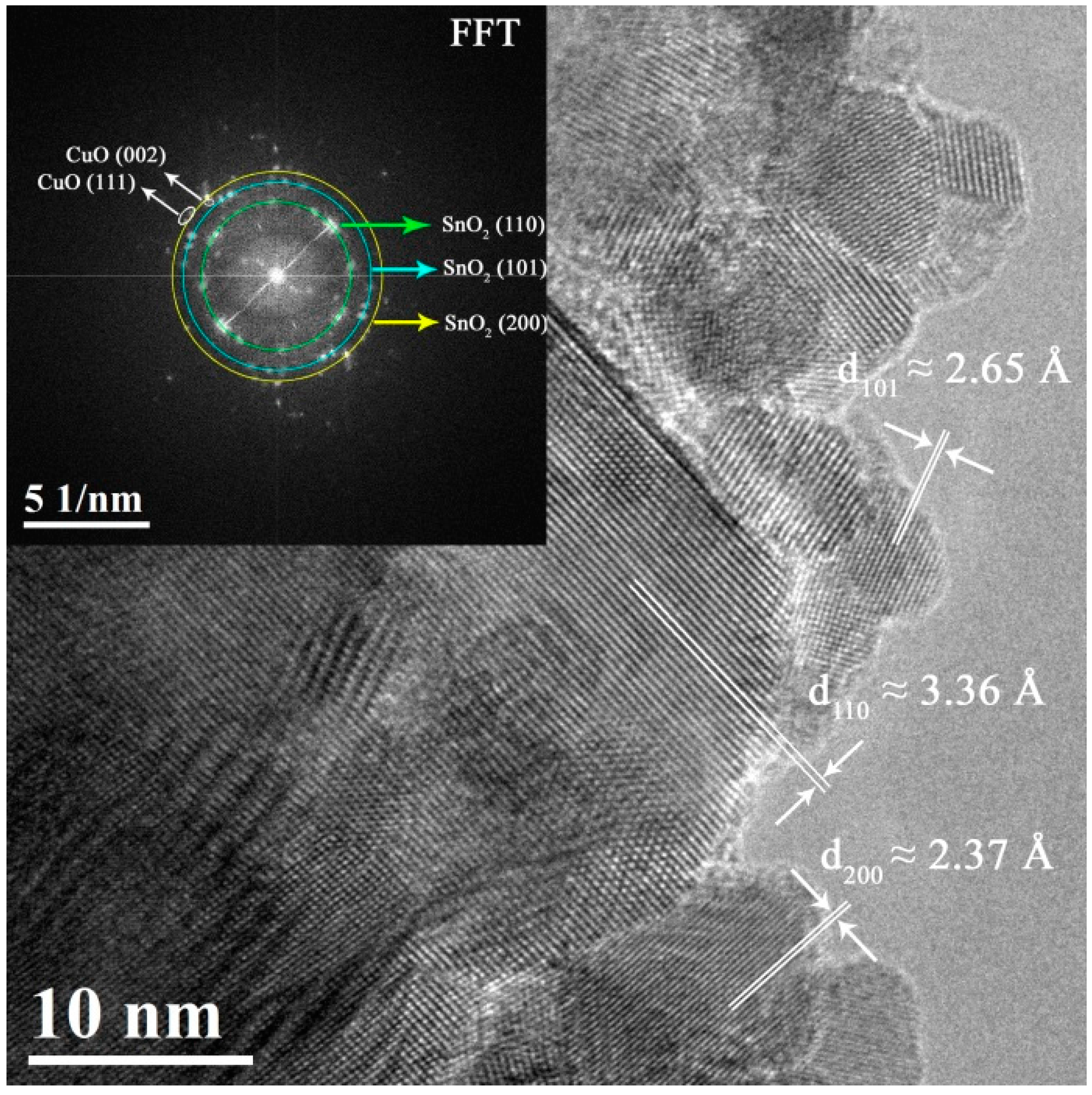
3.1. Morphological and Structural Characterization
3.2. Room and Low Temperature Magnetic Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohno, H. Making Nonmagnetic Semiconductors Ferromagnetic. Science 1998, 281, 951–956. [Google Scholar] [CrossRef]
- Dietl, T. A Ten-Year Perspective on Dilute Magnetic Semiconductors and Oxides. Nat. Mater. 2010, 9, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. A Perspective of Recent Progress in ZnO Diluted Magnetic Semiconductors. Appl. Phys. A Mater. Sci. Process. 2013, 112, 241–254. [Google Scholar] [CrossRef]
- Yamada, Y.; Ueno, K.; Fukumura, T.; Yuan, H.T.; Shimotani, H.; Iwasa, Y.; Gu, L.; Tsukimoto, S.; Ikuhara, Y.; Kawasaki, M. Electrically Induced Ferromagnetism at Room Temperature in Cobalt-Doped Titanium Dioxide. Science 2011, 332, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Weisheit, M.; Fahler, S.; Marty, A.; Souche, Y.; Poinsignon, C.; Givord, D. Electric Field-Induced Modification of Magnetism in Thin-Film Ferromagnets. Science 2007, 315, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Novák, V.; Olejník, K.; Wunderlich, J.; Cukr, M.; Výborný, K.; Rushforth, A.W.; Edmonds, K.W.; Campion, R.P.; Gallagher, B.L.; Sinova, J.; et al. Curie Point Singularity in the Temperature Derivative of Resistivity in (Ga,Mn)As. Phys. Rev. Lett. 2008, 101, 77201. [Google Scholar] [CrossRef] [PubMed]
- Coey, J.M.D. Handbook of Spin Transport and Magnetism; Tsymbal, E.I., Zutic, I., Eds.; Chapman and Hall/CRC: London, UK, 2011; Chapter 20; pp. 405–426. ISBN 9781439803776. [Google Scholar]
- Vachhani, P.S.; Šipr, O.; Bhatnagar, A.K.; Ramamoorthy, R.K.; Choudhary, R.J.; Phase, D.M.; Dalba, G.; Kuzmin, A.; Rocca, F. Local Structure and Magnetization of Ferromagnetic Cu-Doped ZnO Films: No Magnetism at the Dopant? J. Alloys Compd. 2016, 678, 304–311. [Google Scholar] [CrossRef]
- Pereira, L.M.C.; Araújo, J.P.; Van Bael, M.J.; Temst, K.; Vantomme, A. Practical Limits for Detection of Ferromagnetism Using Highly Sensitive Magnetometry Techniques. J. Phys. D Appl. Phys. 2011, 44, 215001. [Google Scholar] [CrossRef]
- Pereira, L.M.C.; Araújo, J.P.; Wahl, U.; Decoster, S.; Van Bael, M.J.; Temst, K.; Vantomme, A. Searching for Room Temperature Ferromagnetism in Transition Metal Implanted ZnO and GaN. J. Appl. Phys. 2013, 113, 023903. [Google Scholar] [CrossRef]
- Kim, D.H.; Yang, J.S.; Lee, K.W.; Bu, S.D.; Noh, T.W.; Oh, S.J.; Kim, Y.W.; Chung, J.S.; Tanaka, H.; Lee, H.Y.; et al. Formation of Co Nanoclusters in Epitaxial Ti0.96Co0.04O2 Thin Films and Their Ferromagnetism. Appl. Phys. Lett. 2002, 81, 2421–2423. [Google Scholar] [CrossRef]
- Keavney, D.J.; Buchholz, D.B.; Ma, Q.; Chang, R.P.H. Where Does the Spin Reside in Ferromagnetic Cu-Doped ZnO? Appl. Phys. Lett. 2007, 91, 12501. [Google Scholar] [CrossRef]
- Hong, N.H.; Ruyter, A.; Prellier, W.; Sakai, J.; Huong, N.T. Magnetism in Ni-Doped SnO2 Thin Films. J. Phys. Condens. Matter 2005, 17, 6533–6538. [Google Scholar] [CrossRef]
- Hu, S.; Yan, S.; Lin, X.; Yao, X.; Chen, Y.; Liu, G.; Mei, L. Electronic Structure of Fe-Doped In2O3 Magnetic Semiconductor with Oxygen Vacancies: Evidence for F-Center Mediated Exchange Interaction. Appl. Phys. Lett. 2007, 91, 262514. [Google Scholar] [CrossRef]
- Hakimi, A.M.H.R.; Schoofs, F.; Bali, R.; Stelmashenko, N.A.; Blamire, M.G.; Langridge, S.; Cavill, S.A.; Van Der Laan, G.; Dhesi, S.S. Origin of Magnetism in Cobalt-Doped Indium Tin Oxide Thin Films. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 82, 1–7. [Google Scholar] [CrossRef]
- Singhal, A.; Achary, S.N.; Manjanna, J.; Jayakumar, O.D.; Kadam, R.M.; Tyagi, A.K. Colloidal Fe-Doped Indium Oxide Nanoparticles: Facile Synthesis, Structural, and Magnetic Properties. J. Phys. Chem. C 2009, 113, 3600–3606. [Google Scholar] [CrossRef]
- Kulkarni, J.S.; Kazakova, O.; Holmes, J.D. Dilute Magnetic Semiconductor Nanowires. Appl. Phys. A 2006, 85, 277–286. [Google Scholar] [CrossRef]
- Pellicer, E.; Cabo, M.; Rossinyol, E.; Solsona, P.; Suriñach, S.; Baró, M.D.; Sort, J. Nanocasting of Mesoporous In-TM (TM=Co, Fe, Mn) Oxides: Towards 3d Diluted-Oxide Magnetic Semiconductor Architectures. Adv. Funct. Mater. 2013, 23, 900–911. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, J.; Solsona, P.; Suriñach, S.; Baró, M.D.; Sort, J.; Pellicer, E. Nanocasting Synthesis of Mesoporous SnO2 with a Tunable Ferromagnetic Response through Ni Loading. RSC Adv. 2016, 6, 104799–104807. [Google Scholar] [CrossRef]
- Jun, Y.; Jung, Y.; Cheon, J. Architectural Control of Magnetic Semiconductor Nanocrystals. J. Am. Chem. Soc. 2002, 124, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Madelung, O. Semiconductors-Basic Data, 2nd ed.; Springer: Berlin, Germany, 1996. [Google Scholar]
- Ganose, A.M.; Scanlon, D.O. Band Gap and Work Function Tailoring of SnO2 for Improved Transparent Conducting Ability in Photovoltaics. J. Mater. Chem. C 2016, 4, 1467–1475. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Rana, A.M.; Bhatti, M.T. Effect of Annealing on Electrical Resistivity of Rf-Magnetron Sputtered Nanostructured SnO2 Thin Films. Appl. Surf. Sci. 2009, 255, 8562–8565. [Google Scholar] [CrossRef]
- Tsokkou, D.; Othonos, A.; Zervos, M. Carrier Dynamics and Conductivity of SnO2 Nanowires Investigated by Time-Resolved Terahertz Spectroscopy. Appl. Phys. Lett. 2012, 100, 133101. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A Comprehensive Review on Structures and Gas Sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, Q.; Xie, Z.; Zheng, L. The Effect of Noble Metal (Au, Pd and Pt) Nanoparticles on the Gas Sensing Performance of SnO2-Based Sensors: A Case Study on the {221} High-Index Faceted SnO2Octahedra. CrystEngComm 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Rebholz, J.; Bonanati, P.; Jaeschke, C.; Hübner, M.; Mädler, L.; Weimar, U.; Barsan, N. Conduction Mechanism in Undoped and Antimony Doped SnO2 Based FSP Gas Sensors. Sens. Actuators B Chem. 2013, 188, 631–636. [Google Scholar] [CrossRef]
- Han, C.; Han, S.; Khatkar, S.P. Enhancement of H2-Sensing Properties of F-Doped SnO2 Sensor by Surface Modification with SiO2. Sensors 2006, 6, 492–502. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. A Facile Nanocasting Synthesis of Mesoporous Ag-Doped SnO2 Nanostructures with Enhanced Humidity Sensing Performance. Sens. Actuators B Chem. 2016, 223, 750–760. [Google Scholar] [CrossRef]
- Li, L.; Lin, H.; Qu, F. Synthesis of Mesoporous SnO2 Nanomaterials with Selective Gas-Sensing Properties. J. Sol-Gel Sci. Technol. 2013, 67, 545–555. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Tan, Z.; Li, Z.; Ding, G.; Jiao, Z.; Gu, P. Preparation of SnO2 nanoparticles by hard template method for high selectivity gas sensors. J. Nanosci. Nanotechnol. 2011, 11, 11023–11027. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.K.; Kong, S.S.; Kim, Y.S.; Lee, J.H.; Park, W.K.; Park, S.C.; Kim, J.M. Solvent-Free Infiltration Method for Mesoporous SnO2 Using Mesoporous Silica Templates. Microporous Mesoporous Mater. 2009, 120, 441–446. [Google Scholar] [CrossRef]
- Brezesinski, T.; Fischer, A.; Iimura, K.; Sanchez, C.; Grosso, D.; Antonietti, M.; Smarsly, B.M. Generation of Self-Assembled 3d Mesostructured SnO2 Thin Films with Highly Crystalline Frameworks. Adv. Funct. Mater. 2006, 16, 1433–1440. [Google Scholar] [CrossRef]
- Manjula, P.; Boppella, R.; Manorama, S.V. A Facile and Green Approach for the Controlled Synthesis of Porous SnO2Nanospheres: Application as an Efficient Photocatalyst and an Excellent Gas Sensing Material. ACS Appl. Mater. Interfaces 2012, 4, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, S.; Liu, J.; He, C.; Lu, G.; Liu, W. Synthesis of Mesoporous SnO2 Spheres and Application in Gas Sensors. Eur. J. Inorg. Chem. 2014, 2014, 863–869. [Google Scholar] [CrossRef]
- Oh, H.-S.; Nong, H.N.; Strasser, P. Preparation of Mesoporous Sb-, F-, and In-Doped SnO2 Bulk Powder with High Surface Area for Use as Catalyst Supports in Electrolytic Cells. Adv. Funct. Mater. 2015, 25, 1074–1081. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Li, Q.; Wang, C.; Chen, A.; Zhang, Z.; Fan, R.; Yin, L. Ordered mesoporous SnO2 with a highly crystalline state as an anode material for lithium ion batteries with enhanced electrochemical performance. CrystEngComm 2013, 15, 3696–3704. [Google Scholar] [CrossRef]
- Park, J.T.; Ahn, S.H.; Roh, D.K.; Lee, C.S.; Kim, J.H. Multifunctional Organized Mesoporous Tin Oxide Films Templated by Graft Copolymers for Dye-Sensitized Solar Cells. ChemSusChem 2014, 7, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, E.; Lee, J. Ordered Mesoporous SnO2-Based Photoanodes for High-Performance Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 22032–22037. [Google Scholar] [CrossRef]
- Lutterotti, L.; Scardi, P. Simultaneous Structure and Size-strain Refinement by the Rietveld Method. J. Appl. Cryst. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- MAUD (Materials Analysis Using Diffraction). Available online: http://maud.radiographema.com/ (accessed on 15 February 2017).
- Gabasch, H.; Kleimenov, E.; Teschner, D.; Zafeiratos, S.; Knop-gericke, A.; Zemlyanov, D.; Aszalos-kiss, B.; Hayek, K. Carbon Incorporation during Ethene Oxidation on Pd(111) Studied by in-Situ X-ray Photoelectron Spectroscopy at 2 × 10−3 Mbar. J. Catal. 2006, 242, 340–348. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation, Physical Electronics Division: Waltham, MA, USA, 1992; Available online: https://www.cnyn.unam.mx/~wencel/XPS/MANXPS.pdf.
- Vlachos, D.; Craven, A.J.; McComb, D.W. Specimen Charging in X-ray Absorption Spectroscopy: Correction of Total Electron Yield Data from Stabilized Zirconia in the Energy Range 250–915 eV. J. Synchrotron Radiat. 2005, 12, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Niimura, N.; Hiraoka, K. Prevention of the Reduction of CuO during X-ray Photoelectron Spectroscopy Analysis. Surf. Interface Anal. 1996, 24, 193–197. [Google Scholar] [CrossRef]
- Espinós, J.P.; Morales, J.; Barranco, A.; Caballero, A.; Holgado, J.P.; González-Elipe, A.R. Interface Effects for Cu, CuO, and Cu2O Deposited on SiO2 and ZrO2. XPS Determination of the Valence State of Copper in Cu/SiO2 and Cu/ZrO2 Catalysts. J. Phys. Chem. B 2002, 106, 6921–6929. [Google Scholar] [CrossRef]
- Diaz-Droguett, D.E.; Espinoza, R.; Fuenzalida, V.M. Copper Nanoparticles Grown under Hydrogen: Study of the Surface Oxide. Appl. Surf. Sci. 2011, 257, 4597–4602. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.; Wang, X.; Zhan, Y.; Liu, Y.; Zheng, C. Synthesis and Characterization of Cu2O Nanowires by a Novel Reduction Route. Adv. Mater. 2002, 14, 67–69. [Google Scholar] [CrossRef]
- Younas, M.; Shen, J.; He, M.; Lortz, R.; Azad, F.; Akhtar, M.J.; Maqsood, A.; Ling, F.C.C. Role of Multivalent Cu, Oxygen Vacancies and CuO Nanophase in the Ferromagnetic Properties of ZnO:Cu Thin Films. RSC Adv. 2015, 5, 55648–55657. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Thissen, A.; Jaegermann, W.; Liu, M. Photoelectron Spectroscopy Study of Oxygen Vacancy on Vanadium Oxides Surface. Appl. Surf. Sci. 2004, 236, 473–478. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Aguilar, T.; Hernández, N.C.; de los Santos, D.M.; Sánchez-Márquez, J.; Zorrilla, D.; Fernández-Lorenzo, C.; Alcántara, R.; Martín-Calleja, J. Experimental and Theoretical Study of the Electronic Properties of Cu-Doped Anatase TiO2. Phys. Chem. Chem. Phys. 2014, 16, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, A.; Magnone, H.; Seehra, M.S.; Bonevich, J. Bulk to Nanoscale Magnetism and Exchange Bias in CuO Nanoparticles. Phys. Rev. B 2001, 64, 174420. [Google Scholar] [CrossRef]
- Danan, H.; Herr, A.; Meyer, A.J.P. New Determinations of the Saturation Magnetization of Nickel and Iron. J. Appl. Phys. 1968, 39, 669–670. [Google Scholar] [CrossRef]
- Wang, Z.; Qureshi, N.; Yasin, S.; Mukhin, A.; Ressouche, E.; Zherlitsyn, S.; Skourski, Y.; Geshev, J.; Ivanov, V.; Gospodinov, M.; et al. Magnetoelectric Effect and Phase Transitions in CuO in External Magnetic Fields. Nat. Commun. 2016, 7, 10295. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.R.; Losby, J.; Dubenko, I.; Roy, S.; Ali, N.; Marasinghe, K. Magnetic Properties of Mechanically Milled Nanosized Cupric Oxide. J. Magn. Magn. Mater. 2004, 279, 111–117. [Google Scholar] [CrossRef]
- Ramazanoglu, M.; Laver, M.; Ratcliff, W.; Watson, S.M.; Chen, W.C.; Jackson, A.; Kothapalli, K.; Lee, S.; Cheong, S.W.; Kiryukhin, V. Local Weak Ferromagnetism in Single-Crystalline Ferroelectric BiFeO3. Phys. Rev. Lett. 2011, 107, 207206. [Google Scholar] [CrossRef] [PubMed]
- Kodama, R.; Makhlouf, S.; Berkowitz, A. Finite Size Effects in Antiferromagnetic NiO Nanoparticles. Phys. Rev. Lett. 1997, 79, 1393–1396. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Hanzo, L., Ed.; Wiley-IEEE Press: Hoboken, NJ, USA, 2008; ISBN 9780471477419. [Google Scholar]
- Benitez, M.J.; Petracic, O.; Salabas, E.L.; Radu, F.; Tüysüz, H.; Schüth, F.; Zabel, H. Evidence for Core-Shell Magnetic Behavior in Antiferromagnetic Co3O4 Nanowires. Phys. Rev. Lett. 2008, 101, 97206. [Google Scholar] [CrossRef] [PubMed]
- Passamani, E.C.; Larica, C.; Marques, C.; Proveti, J.R.; Takeuchi, A.Y.; Sanchez, F.H. Exchange Bias and Anomalous Vertical Shift of the Hysteresis Loops in Milled Fe/MnO2 Material. J. Magn. Magn. Mater. 2006, 299, 11–20. [Google Scholar] [CrossRef]
- Thakur, P.; Bisogni, V.; Cezar, J.C.; Brookes, N.B.; Ghiringhelli, G.; Gautam, S.; Chae, K.H.; Subramanian, M.; Jayavel, R.; Asokan, K. Electronic Structure of Cu-Doped ZnO Thin Films by X-Ray Absorption, Magnetic Circular Dichroism, and Resonant Inelastic X-ray Scattering. J. Appl. Phys. 2010, 107, 103915. [Google Scholar] [CrossRef]
- Chen, J.M.; Chang, S.C.; Liu, R.S.; Lee, J.M.; Park, M.; Choy, J.H. Soft-X-ray Absorption Spectroscopy of Heterostructured High-TC Superconducting Nanohybrids: X-Bi2Sr2CaCu2O8 [X=I, HgI2, and (Py-CH3)2HgI4]. Phys. Rev. B 2005, 71, 94501. [Google Scholar] [CrossRef]
- Mizokawa, T.; Fujimori, A. Electronic Structure of Tetragonal LaCuO3 Studied by Photoemission and X-ray-Absorption Spectroscopy. Phys. Rev. B 1998, 57, 9550–9556. [Google Scholar] [CrossRef]
- Karppinen, M.; Kotiranta, M.; Yamauchi, H.; Nachimuthu, P.; Liu, R.S.; Chen, J.M. O K-Edge and Cu L23-Edge XANES Study on the Concentration and Distribution of Holes in the (Pb2/3Cu1/3)3Sr2(Y,Ca) Cu2O8+z Superconductive Phase. Phys. Rev. B 2001, 63, 184507. [Google Scholar] [CrossRef]
- Menéndez, E.; Demeter, J.; Van Eyken, J.; Nawrocki, P.; Jedryka, E.; Wójcik, M.; Lopez-Barbera, J.F.; Nogués, J.; Vantomme, A.; Temst, K. Improving the Magnetic Properties of Co-CoO Systems by Designed Oxygen Implantation Profiles. ACS Appl. Mater. Interfaces 2013, 5, 4320–4327. [Google Scholar] [CrossRef] [PubMed]







| [Cu(II)]:[Sn(II)] | Cu Content Determined by EDX (at.%) | Crystallite Size SnO2 Phase (nm) (±1) | a (Å) SnO2 Phase (±1 × 10−3) | c (Å) SnO2 Phase (±1 × 10−3) |
|---|---|---|---|---|
| 0:100 | 0 | 9 | 4.737 | 3.187 |
| 5:95 | 1 | 7 | 4.739 | 3.189 |
| 15:85 | 5 | 6 | 4.739 | 3.189 |
| 20:80 | 7 | 7 | 4.737 | 3.186 |
| 5 K | ||||
|---|---|---|---|---|
| 1 at.% Cu | 7 at.% Cu | |||
| 50 kOe | −50 kOe | 50 kOe | −50 kOe | |
| XMCD ± δXMCD | 36 ± 2% | 29 ± 2% | 39 ± 3% | 32 ± 4% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Menéndez, E.; Guerrero, M.; Quintana, A.; Weschke, E.; Pellicer, E.; Sort, J. Unraveling the Origin of Magnetism in Mesoporous Cu-Doped SnO2 Magnetic Semiconductors. Nanomaterials 2017, 7, 348. https://doi.org/10.3390/nano7110348
Fan J, Menéndez E, Guerrero M, Quintana A, Weschke E, Pellicer E, Sort J. Unraveling the Origin of Magnetism in Mesoporous Cu-Doped SnO2 Magnetic Semiconductors. Nanomaterials. 2017; 7(11):348. https://doi.org/10.3390/nano7110348
Chicago/Turabian StyleFan, Junpeng, Enric Menéndez, Miguel Guerrero, Alberto Quintana, Eugen Weschke, Eva Pellicer, and Jordi Sort. 2017. "Unraveling the Origin of Magnetism in Mesoporous Cu-Doped SnO2 Magnetic Semiconductors" Nanomaterials 7, no. 11: 348. https://doi.org/10.3390/nano7110348
APA StyleFan, J., Menéndez, E., Guerrero, M., Quintana, A., Weschke, E., Pellicer, E., & Sort, J. (2017). Unraveling the Origin of Magnetism in Mesoporous Cu-Doped SnO2 Magnetic Semiconductors. Nanomaterials, 7(11), 348. https://doi.org/10.3390/nano7110348






