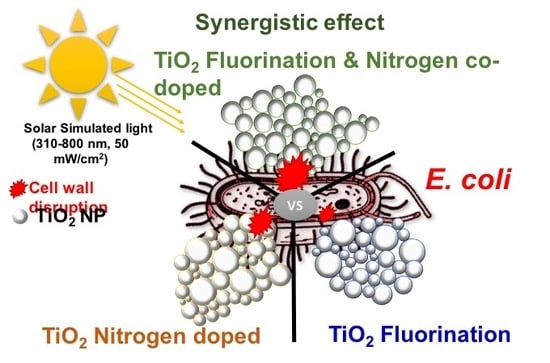
Synergistic Effect of Fluorinated and N Doped TiO2 Nanoparticles Leading to Different Microstructure and Enhanced Photocatalytic Bacterial Inactivation
Abstract
:1. Introduction
2. Results
2.1. Untreated Particles and Effect of Attrition Milling
2.2. Experimental Change upon HT
2.3. Photocatalytic Activity
3. Discussion
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Photocatalytic Activity
4.3. Characterisation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2004. [Google Scholar]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, A.L. What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology 2008, 17, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; Bkc: Tokyo, Japan, 1999; ISBN 4-939051-03-X. [Google Scholar]
- Bruno, T.J.; Svoronos, P.D.N. CRC Handbook of Fundamental Spectroscopic Correlation Charts; CRC Press and Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Luo, J.; Wang, S.; Liu, W.; Tian, C.; Wu, J.; Zu, X.; Zhou, W.; Yuan, X.; Xiang, X. Influence of different aluminum salts on the photocatalytic properties of Al doped TiO2 nanoparticles towards the degradation of AO7 dye. Sci. Rep. 2017, 7, 8108. [Google Scholar] [CrossRef] [PubMed]
- Bloh, J.Z.; Dillert, R.; Bahnemann, D.W. Designing Optimal Metal-Doped Photocatalysts: Correlation between Photocatalytic Activity, Doping Ratio, and Particle Size. J. Phys. Chem. C 2012, 116, 25558–25562. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, C.; Wang, N.; Li, Y.; Ding, H. Stable Ti3+ Self-Doped Anatase-Rutile Mixed TiO2 with Enhanced Visible Light Utilization and Durability. J. Phys. Chem. C 2016, 120, 6116–6124. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Hwang, S.H.; Jeon, S.; Hwang, B.; Jung, J.-Y.; Lee, J.; Park, S.-H.; Jeong, J.-H. Three-dimensional plasmonic Ag/TiO2 nanocomposite architectures on flexible substrates for visible-light photocatalytic activity. Sci. Rep. 2017, 7, 8915. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhao, X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- Ramacharyulu, P.V.R.K.; Nimbalkar, D.B.; Kumar, J.P.; Prasad, G.K.; Ke, S.-C. N-doped, S-doped TiO2 nanocatalysts: Synthesis, characterization and photocatalytic activity in the presence of sunlight. RSC Adv. 2015, 5, 37096–37101. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Guo, X.; Shen, G.; Xiang, J. Synthesis and characterization of N-doped TiO2 loaded onto activated carbon fiber with enhanced visible-light photocatalytic activity. New J. Chem. 2014, 38, 6139–6146. [Google Scholar] [CrossRef]
- Nakano, Y.; Morikawa, T.; Ohwaki, T.; Taga, Y. Deep-level optical spectroscopy investigation of N-doped TiO2 films. Appl. Phys. Lett. 2005, 86, 132104. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yamasue, E.; Okumura, H.; Ishihara, K.N. Effect of oxygen and nitrogen concentration of nitrogen doped TiOx film as photocatalyst prepared by reactive sputtering. Appl. Catal. Gen. 2009, 371, 179–190. [Google Scholar] [CrossRef]
- Li, J.L.; Ma, X.X.; Sun, M.R.; Li, X.M.; Song, Z.L. Fabrication of nitrogen-doped mesoporous TiO2 layer with higher visible photocatalytic activity by plasma-based ion implantation. Thin Solid Films 2010, 519, 101–105. [Google Scholar]
- Premkumar, J. Development of Super-Hydrophilicity on Nitrogen-Doped TiO2 Thin Film Surface by Photoelectrochemical Method under Visible Light. Chem. Mater. 2004, 16, 3980–3981. [Google Scholar] [CrossRef]
- Sarantopoulos, C.; Gleizes, A.N.; Maury, F. Chemical vapor deposition and characterization of nitrogen doped TiO2 thin films on glass substrates. Thin Solid Films 2009, 518, 1299–1303. [Google Scholar] [CrossRef] [Green Version]
- Kafizas, A.; Crick, C.; Parkin, I.P. The combinatorial atmospheric pressure chemical vapour deposition (cAPCVD) of a gradating substitutional/interstitial N-doped anatase TiO2 thin-film; UVA and visible light photocatalytic activities. J. Photochem. Photobiol. Chem. 2010, 216, 156–166. [Google Scholar] [CrossRef]
- Pore, V.; Heikkilä, M.; Ritala, M.; Leskelä, M.; Areva, S. Atomic layer deposition of TiO2−xNx thin films for photocatalytic applications. J. Photochem. Photobiol. Chem. 2006, 177, 68–75. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Q.; Lian, J. Visible-light photocatalytic activity of nitrogen-doped TiO2 thin film prepared by pulsed laser deposition. Appl. Surf. Sci. 2008, 254, 4620–4625. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Senna, M.; Myers, N.; Aimable, A.; Laporte, V.; Pulgarin, C.; Baghriche, O.; Bowen, P. Modification of titania nanoparticles for photocatalytic antibacterial activity via a colloidal route with glycine and subsequent annealing. J. Mater. Res. 2012, 28, 354–361. [Google Scholar] [CrossRef]
- Senna, M.; Sepelak, V.; Shi, J.; Bauer, B.; Feldhoff, A.; Laporte, V.; Becker, K.D. Introduction of oxygen vacancies and fluorine into TiO2 nanoparticles by co-milling with PTFE. J. Solid State Chem. 2012, 187, 51–57. [Google Scholar] [CrossRef]
- Wu, G.; Wen, J.; Nigro, S.; Chen, A. One-step synthesis of N-and F-codoped mesoporous TiO2 photocatalysts with high visible light activity. Nanotechnology 2010, 21, 085701. [Google Scholar] [CrossRef] [PubMed]
- Hasan Nia, M.; Rezaei-Tavirani, M.; Nikoofar, A.R.; Masoumi, H.; Nasr, R.; Hasanzadeh, H.; Jadidi, M.; Shadnush, M. Stabilizing and dispersing methods of TiO2 nanoparticles in biological studies. J. Paramed. Sci. 2015, 6, 2008–4978. [Google Scholar]
- Raj, K.; Viswanathan, B. Effect of surface area, pore volume and particle size of P25 titania on the phase transformation of anatase to rutile. Indian J. Chem.-Sect. A 2009, 48A, 1378–1382. [Google Scholar]
- Iskandar, F.; Nandiyanto, A.B.D.; Yun, K.M.; Hogan, C.J.; Okuyama, K.; Biswas, P. Enhanced Photocatalytic Performance of Brookite TiO2 Macroporous Particles Prepared by Spray Drying with Colloidal Templating. Adv. Mater. 2007, 19, 1408–1412. [Google Scholar] [CrossRef]
- Aimable, A.; Bowen, P. Nanopowder metrology and nanoparticle size measurement: Towards the development and testing of protocols. Process. Appl. Ceram. 2010, 4, 157–166. [Google Scholar] [CrossRef]
- Zong, X.; Xing, Z.; Yu, H.; Chen, Z.; Tang, F.; Zou, J.; Lu, G.Q.; Wang, L. Photocatalytic water oxidation on F, N co-doped TiO2 with dominant exposed {001} facets under visible light. Chem. Commun. 2011, 47, 11742–11744. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pierzchała, K.; Sienkiewicz, A.; Forró, L.; Kiwi, J.; Moser, J.E.; Pulgarin, C. Synthesis, Characterization, and Photocatalytic Activities of Nanoparticulate N, S-Codoped TiO2 Having Different Surface-to-Volume Ratios. J. Phys. Chem. C 2010, 114, 2717–2723. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Zhang, M.; Yang, J.; Zhang, Z. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; Tan, X.; Lian, J. IR and XPS investigation of visible-light photocatalysis—Nitrogen–carbon-doped TiO2 film. Appl. Surf. Sci. 2006, 518, 1988–1994. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Kontos, A.G.; Likodimos, V.; Katsaros, F.; Boukos, N.; Tsoutsou, D.; Dimoulas, A.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Inorganic-organic core-shell titania nanoparticles for efficient visible light activated photocatalysis. Appl. Catal. B Environ. 2013, 130–131, 14–24. [Google Scholar] [CrossRef]
- Bittencourt, C.; Rutar, M.; Umek, P.; Mrzel, A.; Vozel, K.; Arčon, D.; Henzler, K.; Krüger, P.; Guttmann, P. Molecular nitrogen in N-doped TiO2 nanoribbons. RSC Adv. 2015, 5, 23350–23356. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Gu, Y.; Fan, D. Visible-Light Photocatalytic Activity of N-Doped TiO2 Nanotube Arrays on Acephate Degradation. Available online: https://www.hindawi.com/journals/jnm/2015/527070/ (accessed on 12 July 2017).
- Cheng, X.; Yu, X.; Xing, Z. Characterization and mechanism analysis of Mo–N-co-doped TiO2 nano-photocatalyst and its enhanced visible activity. J. Colloid Interface Sci. 2012, 372, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Batalović, K.; Bundaleski, N.; Radaković, J.; Abazović, N.; Mitrić, M.; Silva, R.A.; Savić, M.; Belošević-Čavor, J.; Rakočević, Z.; Rangel, C.M. Modification of N-doped TiO2 photocatalysts using noble metals (Pt, Pd)—A combined XPS and DFT study. Phys. Chem. Chem. Phys. 2017, 19, 7062–7071. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Rahimi, R.; Saadati, S.; Honarvar Fard, E. Fluorine-doped TiO2 nanoparticles sensitized by tetra(4-carboxyphenyl)porphyrin and zinc tetra(4-carboxyphenyl)porphyrin: Preparation, characterization, and evaluation of photocatalytic activity. Environ. Prog. Sustain. Energy 2015, 34, 1341–1348. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- He, X.; Aker, W.G.; Pelaez, M.; Lin, Y.; Dionysiou, D.D.; Hwang, H. Assessment of nitrogen-fluorine-codoped TiO2 under visible light for degradation of BPA: Implication for field remediation. J. Photochem. Photobiol. Chem. 2016, 314, 81–92. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of High-Temperature Stable Anatase TiO2 Photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef] [Green Version]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A Highly Efficient TiO2-xCx Nano-heterojunction Photocatalyst for Visible Light Induced Antibacterial Applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.M.; Naik, B. Synthesis of mesoporous TiO2−xNx spheres by template free homogeneous co-precipitation method and their photo-catalytic activity under visible light illumination. J. Colloid Interface Sci. 2009, 333, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Tobaldi, D.M.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A. Fully quantitative X-ray characterisation of Evonik Aeroxide TiO2 P25®. Mater. Lett. 2014, 122, 345–347. [Google Scholar] [CrossRef]
- Cherian, C.T.; Reddy, M.V.; Magdaleno, T.; Sow, C.-H.; Ramanujachary, K.V.; Rao, G.V.S.; Chowdari, B.V.R. (N,F)-Co-doped TiO2: Synthesis, anatase–rutile conversion and Li-cycling properties. CrystEngComm 2012, 14, 978–986. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef] [PubMed]
- Czoska, A.M.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, G.; Finazzi, E.; Valentin, C.D.; Pacchioni, G. The Nature of Defects in Fluorine-Doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Huang, D.-G.; Liao, S.-J.; Liu, J.-M.; Dang, Z.; Petrik, L. Preparation of visible-light responsive N–F-codoped TiO2 photocatalyst by a sol-gel-solvothermal method. J. Photochem. Photobiol. Chem. 2006, 184, 282–288. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Andrzejczuk, M.; Pulgarin, C.; Kulik, A.; Kiwi, J. Innovative transparent non-scattering TiO2 bactericide thin films inducing increased E. coli cell wall fluidity. Surf. Coat. Technol. 2014, 254, 333–343. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Czoska, A.M.; Paganini, M.C.; Giamello, E. Density Functional Theory and Electron Paramagnetic Resonance Study on the Effect of N–F Codoping of TiO2. Chem. Mater. 2008, 20, 3706–3714. [Google Scholar] [CrossRef]
- Brauer, J.I.; Szulczewski, G. Important Role of Surface Fluoride in Nitrogen-Doped TiO2 Nanoparticles with Visible Light Photocatalytic Activity. J. Phys. Chem. B 2014, 118, 14188–14195. [Google Scholar] [CrossRef] [PubMed]







| Medium | Sample | Malvern | BET | Fag | ||||
|---|---|---|---|---|---|---|---|---|
| pH | ζ Potential [mV] | DV50 [μm] | Span | SSA [m2.g−1] | dBET [nm] | |||
| 10−3 N HNO3 Solution | P25-UN | 2 | - | 2.43 | 1.72 | 39.7 | 35 | 69 |
| Saline buffer solution | P25-UN | 7 | −5.1 | 3.28 | 2.17 | 39.7 | 35 | 94 |
| P25-N-Att | 7 | −9.9 | - | - | - | - | - | |
| P25-N-HT | 7 | −15.1 | 20.33 | 4.01 | 49.5 | 28 | 726 | |
| P25-F-Att | 7 | −5.3 | - | - | - | - | - | |
| P25-F-HT | 7 | −21.9 | 15.19 | 4.51 | 23.3 | 60 | 253 | |
| P25-F&N-Att | 7 | −5.9 | - | - | - | - | - | |
| P25-F&N-HT | 7 | −20.6 | 21.03 | 4.49 | 20.0 | 70 | 300 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosevic, I.; Jayaprakash, A.; Greenwood, B.; Van Driel, B.; Rtimi, S.; Bowen, P. Synergistic Effect of Fluorinated and N Doped TiO2 Nanoparticles Leading to Different Microstructure and Enhanced Photocatalytic Bacterial Inactivation. Nanomaterials 2017, 7, 391. https://doi.org/10.3390/nano7110391
Milosevic I, Jayaprakash A, Greenwood B, Van Driel B, Rtimi S, Bowen P. Synergistic Effect of Fluorinated and N Doped TiO2 Nanoparticles Leading to Different Microstructure and Enhanced Photocatalytic Bacterial Inactivation. Nanomaterials. 2017; 7(11):391. https://doi.org/10.3390/nano7110391
Chicago/Turabian StyleMilosevic, Irena, Amarnath Jayaprakash, Brigitte Greenwood, Birgit Van Driel, Sami Rtimi, and Paul Bowen. 2017. "Synergistic Effect of Fluorinated and N Doped TiO2 Nanoparticles Leading to Different Microstructure and Enhanced Photocatalytic Bacterial Inactivation" Nanomaterials 7, no. 11: 391. https://doi.org/10.3390/nano7110391
APA StyleMilosevic, I., Jayaprakash, A., Greenwood, B., Van Driel, B., Rtimi, S., & Bowen, P. (2017). Synergistic Effect of Fluorinated and N Doped TiO2 Nanoparticles Leading to Different Microstructure and Enhanced Photocatalytic Bacterial Inactivation. Nanomaterials, 7(11), 391. https://doi.org/10.3390/nano7110391