Effect of Graphene Oxide (GO) on the Morphology and Microstructure of Cement Hydration Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mix Proportions
2.1.1. Materials
2.1.2. Preparation of Cement Mortar with GO
2.2. Testing Procedures
3. Results and Discussion
3.1. Compressive and Flexural Strength of Cement Mortar
3.2. Mechanism Analysis of GO Modified Cementitious Material
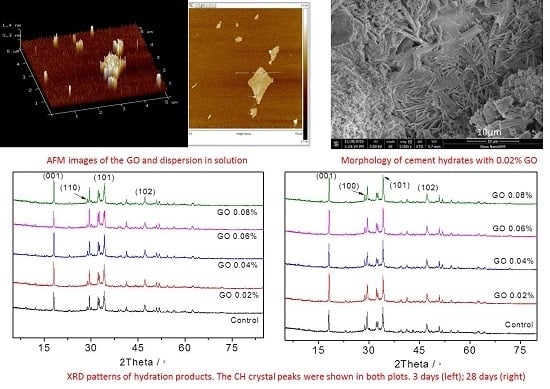
3.3. The Content and Crystallization of Ca(OH)2 in Cement Hydration Products
4. Conclusions and Recommendations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, S.; Barroso-Bujans, F.; Alegría, Á.; Colmenero, J. Dynamics of Water Intercalated in Graphite Oxide. J. Phys. Chem. C 2010, 114, 2604–2612. [Google Scholar] [CrossRef]
- Lin, C.; Wei, W.; Hu, Y.H. Catalytic behavior of graphene oxide for cement hydration process. J. Phys. Chem. Solids 2016, 89, 128–133. [Google Scholar] [CrossRef]
- Tong, T.; Fan, Z.; Liu, Q.; Wang, S.; Tan, S.; Yu, Q. Investigation of the effects of graphene and graphene oxide nanoplatelets on the micro- and macro-properties of cementitious materials. Constr. Build. Mater. 2016, 106, 102–114. [Google Scholar] [CrossRef]
- Yang, H.B.; Manuel, M.; Cui, H.Z.; Han, N.X. Experimental study of the effects of graphene oxide on microstructure and properties of cement paste composite. Compos. Part A 2017, 102, 263–272. [Google Scholar] [CrossRef]
- Li, X.Y.; Yan, M.L.; Wen, G.L.; Chen, Y.L.; Jay, G.S.; Wen, H.D.; Li, Z.J. Effects of graphene oxide agglomerates on workability, hydration, microstructure and compressive strength of cement paste. Constr. Build. Mater. 2017, 145, 402–410. [Google Scholar] [CrossRef]
- Zhong, J.; Zhou, G.X.; He, P.G.; Yang, Z.H.; Jia, D.C. 3D printing strong and conductive geo-polymer nanocomposite structures modified by graphene oxide. Carbon 2017, 117, 421–426. [Google Scholar] [CrossRef]
- Zhu, X.H.; Kang, X.J.; Kai, Y.; Chang, H.Y. Effect of graphene oxide on the mechanical properties and the formation of layered double hydroxides (LDHs) in alkali-activated slag cement. Constr. Build. Mater. 2017, 132, 290–295. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Hou, D.S.; Ma, H.Y.; Fan, T.Y.; Li, Z.J. Effects of graphene oxide on the properties and miceostructures of the magnesium potassium phosphate cement paste. Constr. Build. Mater. 2016, 119, 107–112. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Qin, H.; Hu, Y.H.; Lin, C.; Wei, W.; Hu, Y.H.; Lu, Z.; Hou, D.; Meng, L.; et al. Co-effects of graphene oxide sheets and single wall carbon nanotubes on mechanical properties of cement. J. Phys. Chem. Solids 2015, 30, 39–43. [Google Scholar] [CrossRef]
- Zubir, M.N.M.; Badarudin, A.; Kazi, S.N.; Ming, H.N.; Sadri, R.; Amiri, A. Investigation on the Use of Graphene Oxide as Novel Surfactant for Stabilizing Carbon Based Materials. J. Dispers. Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Gong, K.; Pan, Z.; Korayem, A.H.; Qiu, L.; Li, D. Reinforcing Effects of Graphene Oxide on Portland Cement Paste. J. Mater. Civ. Eng. 2015, 27, A4014010. [Google Scholar] [CrossRef]
- Makar, J. The Effect of SWCNT and Other Nanomaterials on Cement Hydration and Reinforcement. Nanotechnol. Civ. Infrastruct. 2011, 103–130. [Google Scholar]
- Lu, C.; Lu, Z.; Li, Z.; Leung, C.K.Y. Effect of graphene oxide on the mechanical behavior of strain hardening cementitious composites. Constr. Build. Mater. 2016, 120, 457–464. [Google Scholar] [CrossRef]
- Cui, H.; Yan, X.; Tang, L.; Xing, F. Possible pitfall in sample preparation for SEM analysis—A discussion of the paper “Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites” by Lv et al. Cem. Concr. Compos. 2017, 77, 81–85. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, R.; Cai, Y.; Cheng, X.; Shah, S.P. In situ Ca (OH)2 consumption of TEOS on the surface of hardened cement-based materials and its improving effects on the Ca-leaching and sulfate-attack resistivity. Constr. Build. Mater. 2016, 113, 890–896. [Google Scholar] [CrossRef]
- Esteves, L.P. On the hydration of water-entrained cement-silica systems: Combined SEM, XRD and thermal analysis in cement pastes. Thermochim. Acta 2011, 518, 27–35. [Google Scholar] [CrossRef]
- Mounanga, P.; Khelidj, A.; Loukili, A.; Baroghel-Bouny, V. Predicting Ca(OH)2 content and chemical shrinkage of hydrating cement pastes using analytical approach. Cem. Concr. Res. 2004, 34, 255–265. [Google Scholar] [CrossRef]
- Gong, K.; Asce, S.M.; Pan, Z.; Korayem, A.H.; Qiu, L.; Li, D.; Collins, F.; Wang, C.M.; Duan, W.H. Reinforcing Effects of Graphene Oxide on Portland Cement Paste. J. Mater. Civ. Eng. 2014, 27, 1–6. [Google Scholar] [CrossRef]
- Harutyunyan, V.S.; Kirchheim, A.P.; Monteiro, P.J.M.; Aivazyan, A.P.; Fischer, P. Investigation of early growth of calcium hydroxide crystals in cement solution by soft X-ray transmission microscopy. J. Mater. Sci. 2009, 44, 962–969. [Google Scholar] [CrossRef]
- Chen, J.J.; Sorelli, L.; Vandamme, M.; Ulm, F.J.; Chanvillard, G. A Coupled Nanoindentation/SEM-EDS Study on Low Water/Cement Ratio Portland Cement Paste: Evidence for C–S–H/Ca(OH)2 Nanocomposites. J. Am. Ceram. Soc. 2010. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]






| Type | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | Ignition Loss |
|---|---|---|---|---|---|---|---|---|
| PII 52.5 N | 64.95 | 18.31 | 4.21 | 2.95 | 4.22 | 0.64 | 0.788 | 3.21 |
| hkl | GO (%) | 2θ (°) | Peak Height | Crystal Size (nm) | hkl | GO (%) | 2θ (°) | Peak Height | Crystal Size (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 001 | 0 | 18.021 | 1517 | 57.2 | 101 | 0 | 34.080 | 1711 | 21.4 |
| 0.02 | 18.021 | 1909 | 66.4 | 0.02 | 34.080 | 1907 | 28.6 | ||
| 0.04 | 18.080 | 1928 | 61.4 | 0.04 | 34.121 | 1975 | 29.4 | ||
| 0.06 | 18.019 | 1789 | 62.7 | 0.06 | 34.061 | 1852 | 31.0 | ||
| 0.08 | 18.059 | 1929 | 64.9 | 0.08 | 34.100 | 1861 | 26.4 | ||
| 100 | 0 | 28.660 | 368 | 51.4 | 102 | 0 | 47.100 | 647 | 24.4 |
| 0.02 | 28.679 | 411 | 56.3 | 0.02 | 47.099 | 711 | 29.2 | ||
| 0.04 | 28.719 | 407 | 68.4 | 0.04 | 47.159 | 718 | 27 | ||
| 0.06 | 28.659 | 404 | 55.4 | 0.06 | 47.080 | 657 | 26 | ||
| 0.08 | 28.699 | 371 | 64 | 0.08 | 47.120 | 677 | 26.3 |
| hkl | GO (%) | 2θ (°) | Peak Height | Crystal Size (nm) | hkl | GO (%) | 2θ (°) | Peak Height | Crystal Size (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 001 | 0 | 18.039 | 1725 | 59.2 | 101 | 0 | 34.099 | 2040 | 29.7 |
| 0.02 | 18.098 | 2254 | 67.6 | 0.02 | 34.140 | 2211 | 32.8 | ||
| 0.04 | 18.059 | 1867 | 63.4 | 0.04 | 34.101 | 2281 | 40.9 | ||
| 0.06 | 18.100 | 1922 | 67.2 | 0.06 | 34.141 | 2301 | 34.9 | ||
| 0.08 | 18.100 | 1944 | 67.1 | 0.08 | 34.141 | 2287 | 34.4 | ||
| 100 | 0 | 28.68 | 509 | 60.9 | 102 | 0 | 47.120 | 766 | 28.9 |
| 0.02 | 28.739 | 518 | 47.8 | 0.02 | 47.160 | 775 | 27.6 | ||
| 0.04 | 28.698 | 532 | 67.9 | 0.04 | 47.121 | 802 | 29.9 | ||
| 0.06 | 28.740 | 533 | 49.2 | 0.06 | 47.180 | 806 | 29.7 | ||
| 0.08 | 28.739 | 525 | 61.3 | 0.08 | 47.161 | 789 | 30.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, S.; Zheng, D.; Yang, H.; Cui, H.; Tang, W.; Li, D. Effect of Graphene Oxide (GO) on the Morphology and Microstructure of Cement Hydration Products. Nanomaterials 2017, 7, 429. https://doi.org/10.3390/nano7120429
Wang L, Zhang S, Zheng D, Yang H, Cui H, Tang W, Li D. Effect of Graphene Oxide (GO) on the Morphology and Microstructure of Cement Hydration Products. Nanomaterials. 2017; 7(12):429. https://doi.org/10.3390/nano7120429
Chicago/Turabian StyleWang, Liguo, Shupeng Zhang, Dapeng Zheng, Haibin Yang, Hongzhi Cui, Waiching Tang, and Dongxu Li. 2017. "Effect of Graphene Oxide (GO) on the Morphology and Microstructure of Cement Hydration Products" Nanomaterials 7, no. 12: 429. https://doi.org/10.3390/nano7120429
APA StyleWang, L., Zhang, S., Zheng, D., Yang, H., Cui, H., Tang, W., & Li, D. (2017). Effect of Graphene Oxide (GO) on the Morphology and Microstructure of Cement Hydration Products. Nanomaterials, 7(12), 429. https://doi.org/10.3390/nano7120429