Abstract
Earth-abundant and low-cost catalysts with excellent electrocatalytic hydrogen evolution reaction (HER) activity in alkaline solution play an important role in the sustainable production of hydrogen energy. In this work, a catalyst of Ni(P, O)x·MoOx nanowire array on nickel foam has been prepared via a facile route for efficient alkaline HER. Benefiting from the collaborative advantages of Ni(P, O)x and amorphous MoOx, as well as three-dimensional porous conductive nickel scaffold, the hybrid electrocatalyst shows high catalytic activity in 1 M KOH aqueous solution, including a small overpotential of 59 mV at 10 mA cm−2, a low Tafel slope of 54 mV dec-1, and excellent cycling stability.
1. Introduction
Exploring new sustainable energy resources and clean energy carriers to replace the traditional fossil fuels is one of the most important challenges of the 21st century. Hydrogen is considered as the most promising energy carrier for sustainable energy applications due to its outstanding energy storage density, environmental friendliness, and renewability [1,2,3]. Electrochemical water splitting is an important component of several hydrogen generation strategies. However, an efficient catalyst is required to reduce the energy barrier of the hydrogen evolution reaction (HER) [4]. So far, the most effective electrocatalysts for HER are Pt group materials, but the scarcity and high cost of these noble metals significantly limit their wide utilization [5]. Herein, the development of low-cost and efficient HER electrocatalysts based on earth-abundant species is of great importance [6,7,8].
Up to now, various non-precious metal-based materials (e.g., Ni, Co, Fe, Cu, W, and Mo) have been intensively synthesized as promising HER catalysts with high performance [9,10,11,12]. Among these alternatives, crystalline MoO2 has been identified as an excellent candidate owing to its good electric conductivity and high electrocatalytic activity [13,14,15]. It is noted that most of the reported non-precious electrocatalysts are based on crystalline compounds. In recent years, a growing class of amorphous materials have emerged as more efficient electrocatalysts compared with their crystalline counterparts [16,17,18,19,20]. However, the amorphous catalysts suffer from poor cycling stability caused by slow dissolution of the catalyst components during long-term test, thus resulting in easy degradation in the electro-activity [21,22]. To mitigate this critical problem, a large number of studies have shown that coupling different functional species can generate a strong synergistic effect to significantly improve the performance [22]. It is important to note that Ni-based electrocatalysts exhibit excellent HER catalytic activity in alkaline media due to the appropriate OH-Ni2+δ (0 ≤ δ ≤ 1.5) bond strength [23,24]. Therefore, it is highly desirable and interesting to combine Ni-species with MoO2 to synergistically achieve substantial improvements in electro-activity and durability.
Herein, we highlight a Ni(P, O)x·MoOx nanowire array which grows directly on a nickel foam support (Ni(P, O)x·MoOx NA/NF) for a highly efficient electrocatalyst which exhibits preferable HER activity. The direct integration of nanowire array onto the Ni foam not only simplifies the electrode preparation processes, but also ensures the tight connection between electrode framework and active species, resulting in enhanced mechanical stability. In addition, the commercial nickel foam acts as a three-dimensional (3D) macroporous conductive substrate that facilitates facile charge transfer, electrolyte diffusion, and gas bubble release. Consequently, benefiting from the collaborative advantages of Ni-species and amorphous MoOx, the as-prepared Ni(P, O)x·MoOx NA/NF electrode shows a remarkable electrocatalytic activity with a low overpotential of 59 mV to attain a current density of 10 mA cm−2 and superior stability for at least 24 h in an alkaline environment, thereby demonstrating a highly-efficient HER catalyst.
2. Results and Discussion
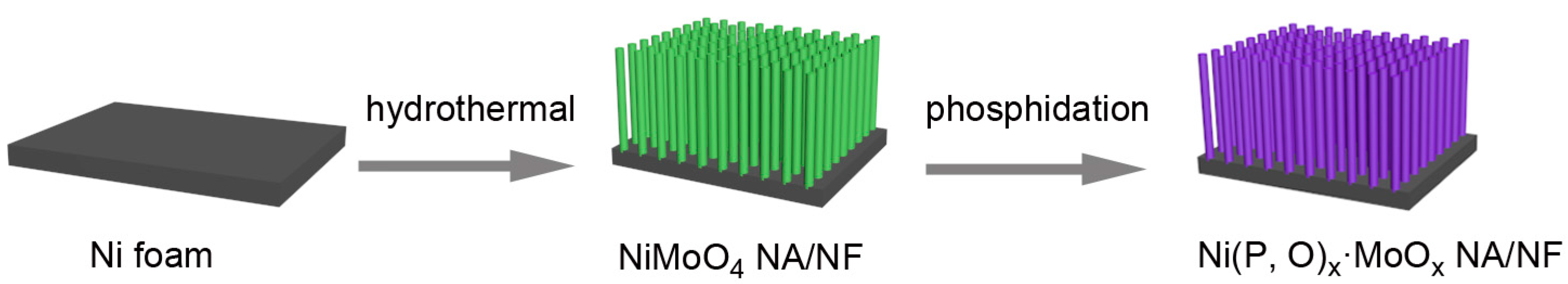
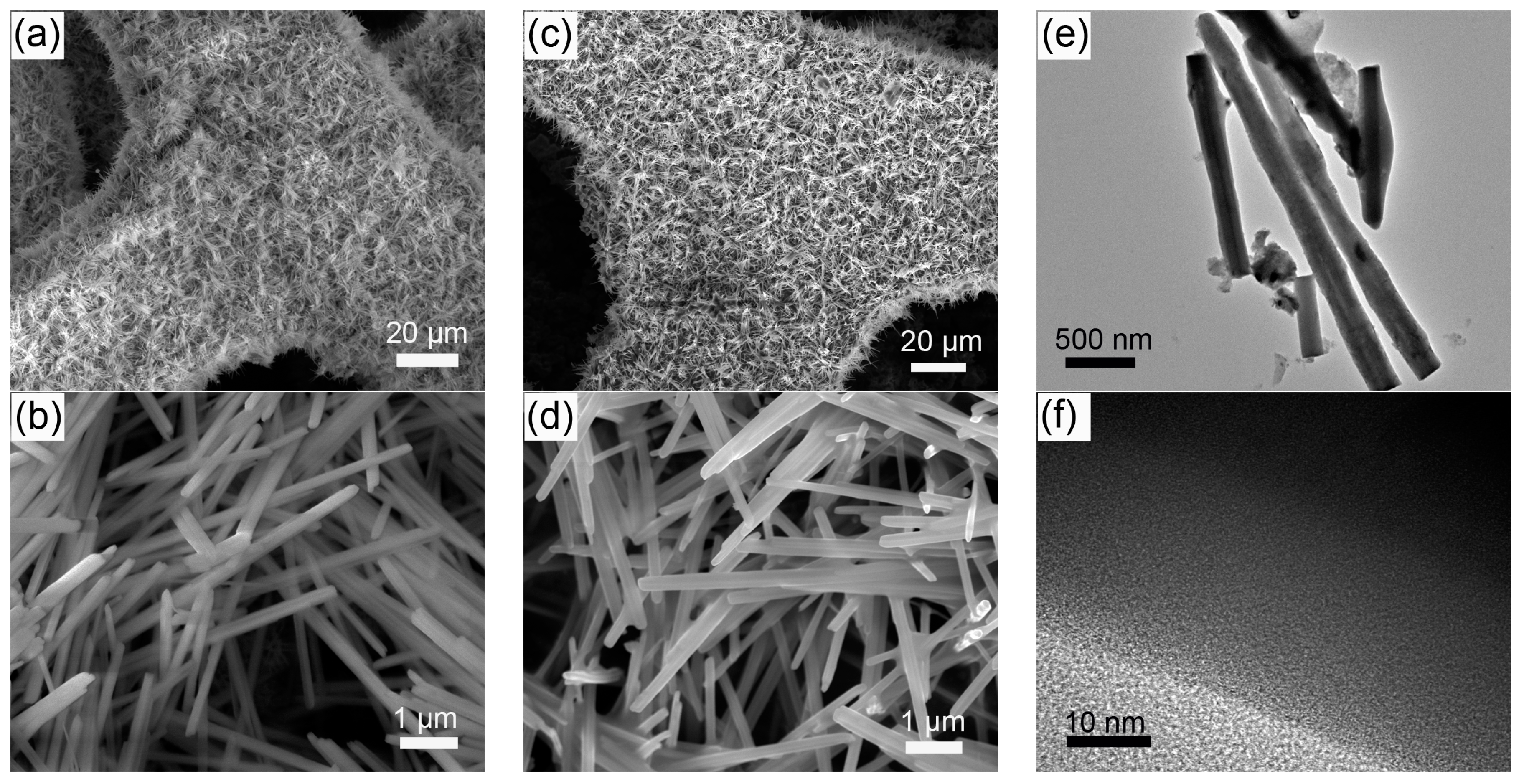
The self-supported Ni(P, O)x·MoOx nanowire array is fabricated on a commercial Ni foam by a facile template-free hydrothermal process in combination with a subsequent in situ phosphorization treatment. Figure 1 schematically illustrates the typical two-step preparation process. In the first step, the Ni(P, O)x·MoOx precursor (i.e., NiMoO4·xH2O) is grown on the 3D porous skeletons of the nickel foam by a hydrothermal reaction. In the second step, the Ni(P, O)x·MoOx catalyst is obtained through a solid-state phosphorization process between the NiMoO4·xH2O precursor and NaH2PO2. The precursor is thermally transformed to crystalline NiMoO4 nanowire array supported on the Ni foam (NiMoO4 NA/NF), during which a simple dehydration reaction occurs. As can be seen from the scanning electron microscopy (SEM) image (Figure 2a), high-density NiMoO4 NA spreads uniformly over the nickel foam skeletons. A closer observation (Figure 2b) indicates that the diameter of the nanowire is about 210 nm, and the length is more than 6 μm. After phosphidation, the 1D nanowire array is maintained well from the precursors (Figure 2c,d), and the diameter of the Ni(P, O)x·MoOx nanowires is similar to the NiMoO4. Transmission electron microscopy (TEM) was employed to further depict the as-prepared Ni(P, O)x·MoOx. Figure 2e shows the corresponding TEM image of Ni(P, O)x·MoOx NA/NF, further identifying the preservation of the 1D morphology after phosphidation. The high-resolution TEM (HRTEM) image (Figure 2f) shows no obvious evidence of lattice fringes, suggesting that the as-synthesized Ni(P, O)x·MoOx is amorphous or of poor crystallinity.
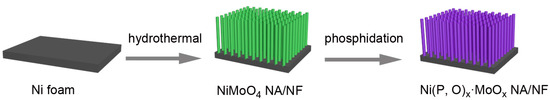
Figure 1.
Schematic illustration of the formation processes of the Ni(P, O)x·MoOx nanowire array which grows directly on a nickel foam support (Ni(P, O)x·MoOx NA/NF).
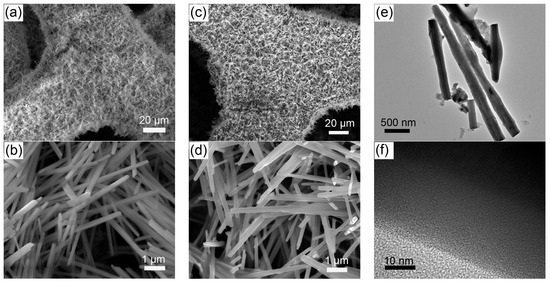
Figure 2.
SEM images of (a,b) NiMoO4 NA/NF and (c,d) Ni(P, O)x·MoOx NA/NF; (e) TEM and (f) HRTEM images of Ni(P, O)x·MoOx NA/NF.
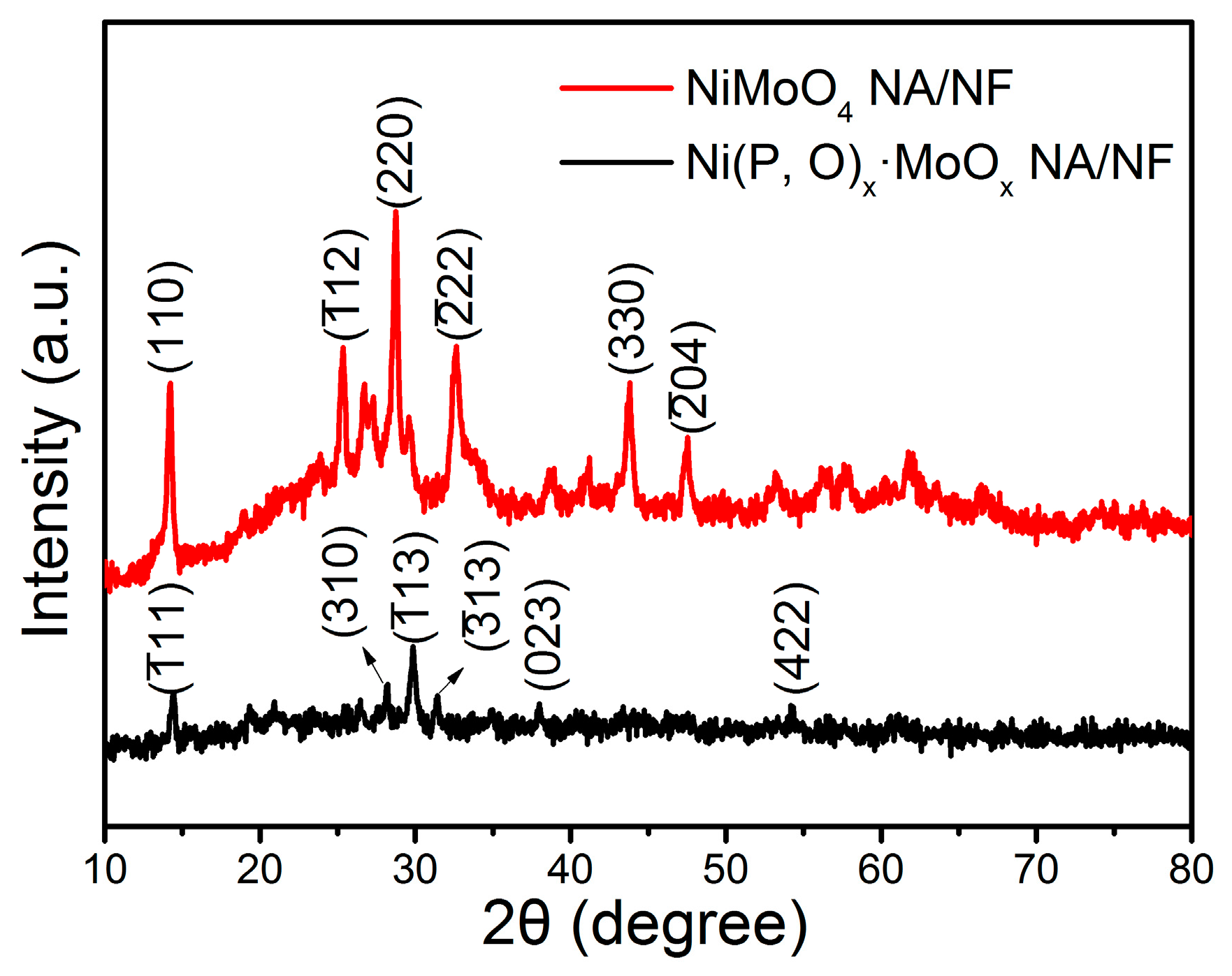
The phase structure of the as-prepared samples was examined by X-ray diffraction (XRD) analysis. As shown in Figure 3, the distinct diffraction peaks with 2θ at around 14.3°, 25.4°, 28.9°, 32.6°, 43.9°, and 47.5° correspond to the (002), (), (220), (), (330), and () crystal planes of NiMoO4, respectively (JCPDS No. 86-0361) [25,26]. Compared with the crystalline NiMoO4, the Ni(P, O)x·MoOx sample exhibits weak diffraction peaks, indicating that the phosphidation process results in a significant decrease in the crystallinity. The main peaks can be assigned to nickel phosphates (Ni2P4O12, JCPDS No. 76-1557). The absence of Mo-related peaks demonstrates that the Mo-based species are amorphous in the as-synthesized Ni(P, O)x·MoOx NA/NF [27].
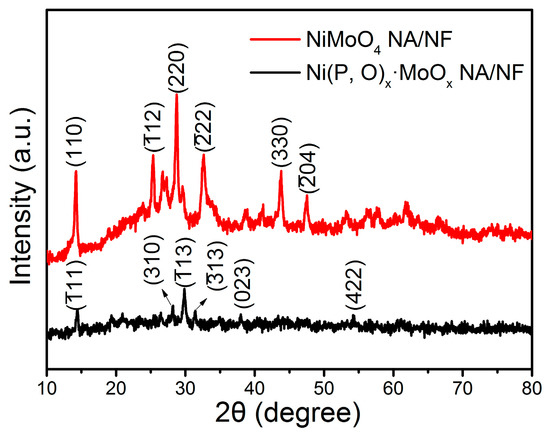
Figure 3.
XRD pattern of the NiMoO4 NA/NF and Ni(P, O)x·MoOx NA/NF.
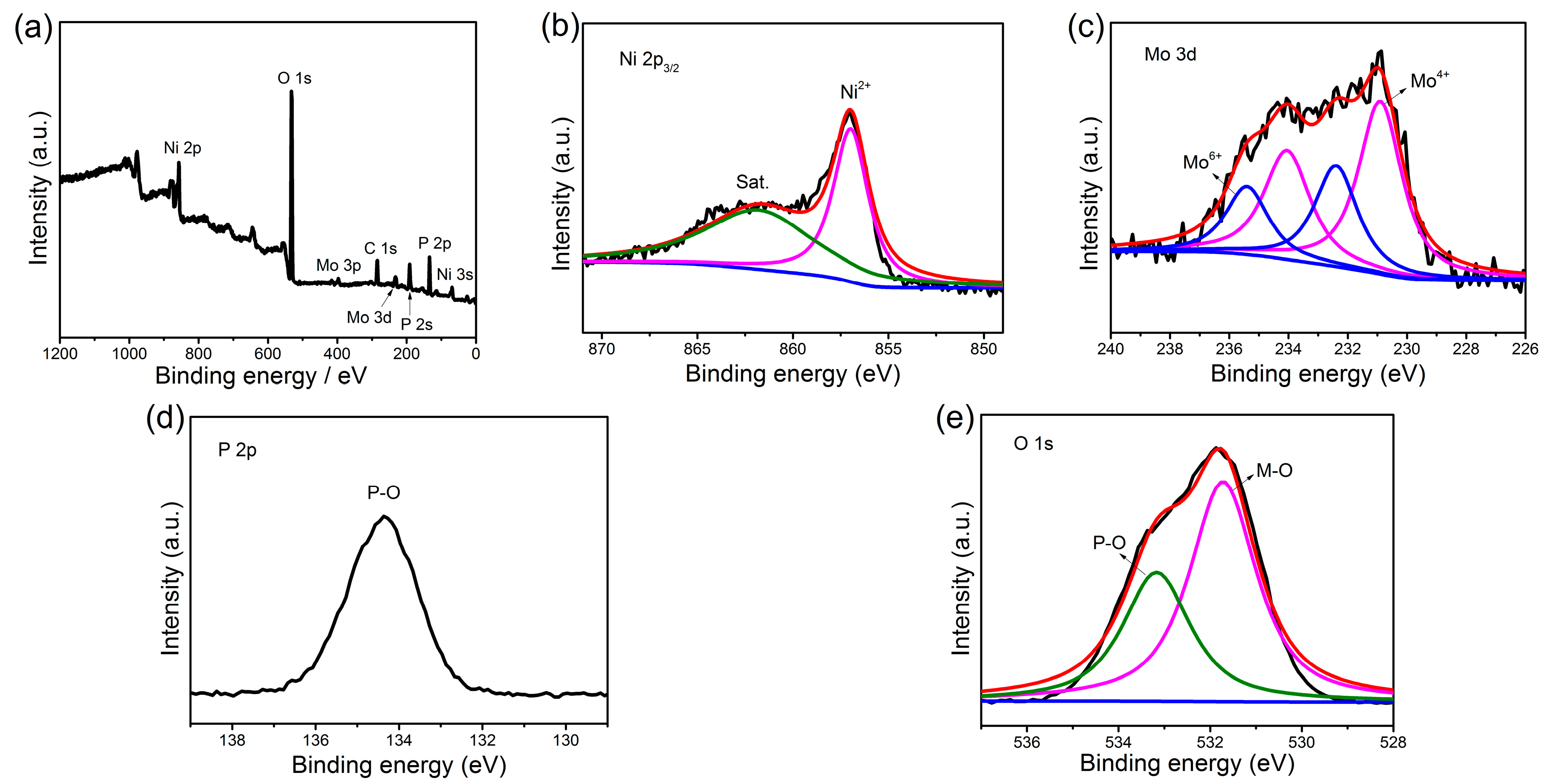
X-ray photoelectron spectroscopy (XPS) measurement was carried out to investigate the surface composition and the oxidation state of the Ni(P, O)x·MoOx NA/NF. The survey spectra show that the Ni(P, O)x·MoOx NA/NF is composed of Mo, Ni, P, O elements (Figure 4a) and the atomic percentage of P in the product is 17.16%. The Ni 2p3/2 high-resolution spectrum (Figure 4b) exhibits two main peaks at binding energies of 856.9 and 862.1 eV, which can be assigned to the Ni–O bond and the satellite peak, respectively [28]. The Mo 3d spectrum of the Ni(P, O)x·MoOx NA/NF (Figure 4c) can be resolved into two sets of peaks corresponding to the Mo6+ and Mo4+ species, and the ratio between the Mo6+ and Mo4+ in the composite is 0.57. The presence of Mo4+ species is probably attributed to the reduction of the Mo6+ precursor during phosphidation process [29]. For the profile of P 2p, the sample (Figure 4d) only shows a peak at a binding energy of 134.4 eV, which represents the P–O bond [30]. The high-resolution O 1s spectrum (Figure 4e) can be fitted into two peaks at 531.7 and 533.1 eV, which can be ascribed to the metal–oxygen (M–O) and P–O bonds, respectively [28].
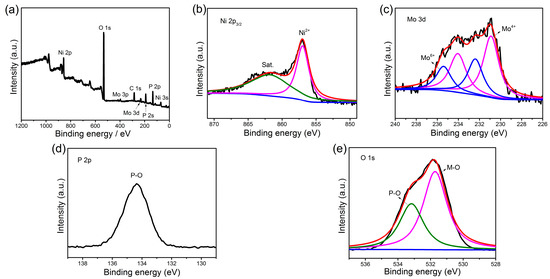
Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of Ni(P, O)x·MoOx NA/NF: (a) full scan; (b) Ni 2p3/2; (c) Mo 3d; (d) P 2p; and (e) O 1s. M–O: metal–oxygen.
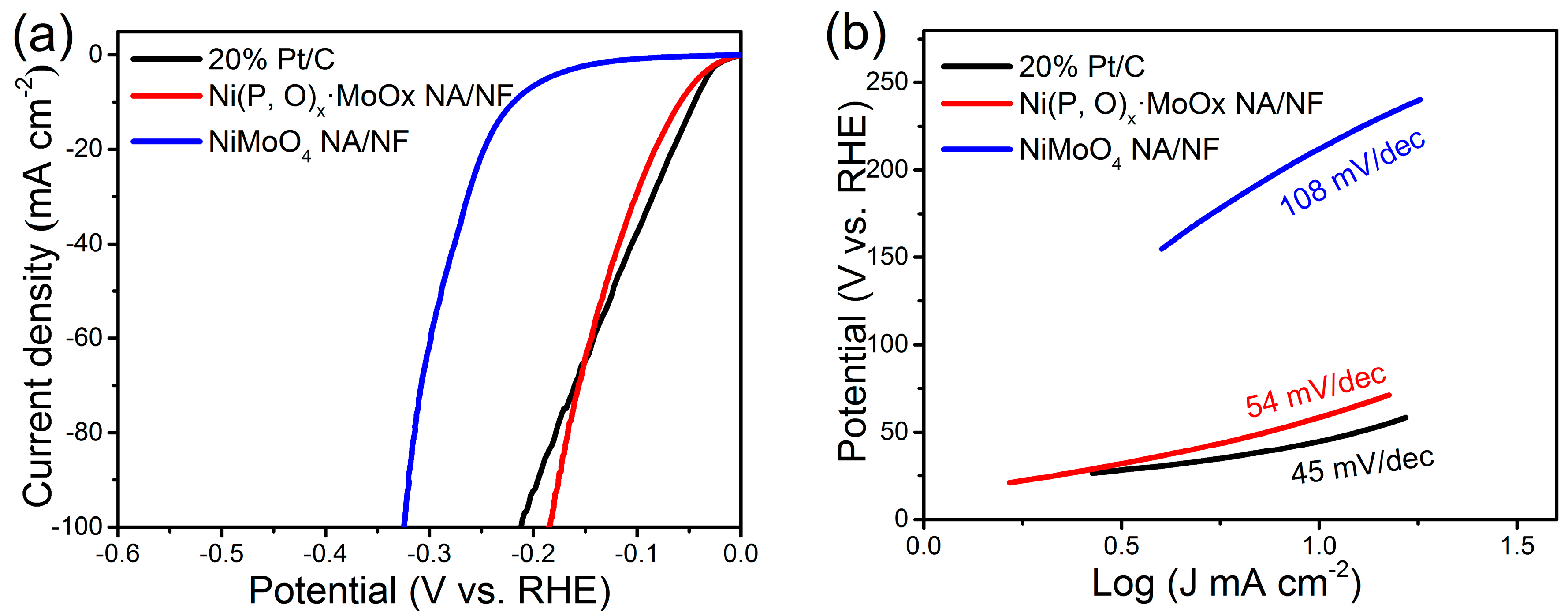
The HER performance of the Ni(P, O)x·MoOx NA/NF was examined in 1 M KOH aqueous solution. For comparison, commercial Pt/C (20 wt % Pt/XC-72) and NiMoO4 NA/NF were also evaluated. Figure 5a shows the IR-corrected linear sweep voltammetry (LSV) curves. The Ni(P, O)x·MoOx NA/NF electrode exhibits a low overpotential of 59 and 185 mV to reach a current density of 10 and 100 mA cm−2, respectively. In sharp contrast, the control NiMoO4 NA/NF electrode requires much higher overpotentials of 219 and 324 mV to achieve the same current densities. The lower overpotential of the Ni(P, O)x·MoOx NA/NF electrode indicates a significant improvement in the HER catalytic property. Impressively, the overpotential is almost comparable to the commercial Pt/C electrode, demonstrating that the present electrode material may serve as a practical cathode for the high-efficiency production of hydrogen.
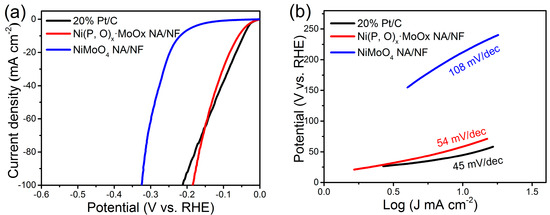
Figure 5.
(a) Linear sweep voltammetry (LSV) polarization curves for hydrogen evolution reaction (HER) and (b) corresponding Tafel plots.
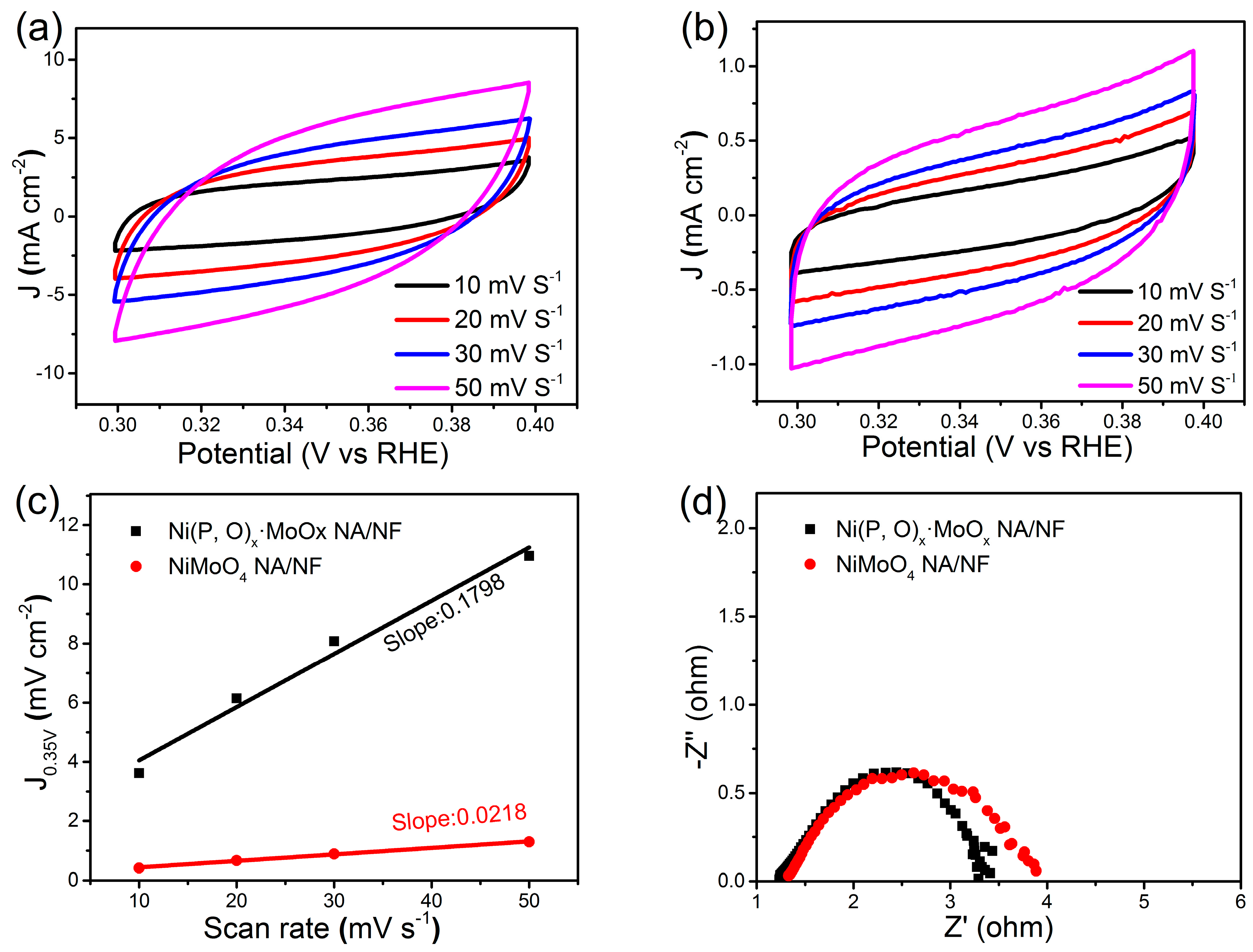
Figure 5b shows the corresponding Tafel plots. It is worth noting that the Tafel slope of Ni(P, O)x·MoOx NA/NF is about 54 mV dec−1, which is only half of the control NiMoO4 NA/NF electrode (108 mV dec−1). This low Tafel slope indicates that the HER occurs on the Ni(P, O)x·MoOx NA/NF electrode following the Volmer–Heyrovsky mechanism, and the rate-limiting step is the electrochemical recombination with an additional proton [9]. More importantly, the Ni(P, O)x·MoOx NA/NF catalytic activity is superior to most Mo-based HER electrocatalysts reported so far (Table 1). In addition, the amount of catalytically active surface area on NiMoO4 NA/NF and Ni(P, O)x·MoOx NA/NF electrodes are roughly estimated from the electrochemical double-layer capacitance (Cdl) by measuring cyclic voltammetry (CV) curves at different scanning rates (Figure 6a,b). The determined Cdl for Ni(P, O)x·MoOx NA/NF (89.9 mF cm−2) is much higher than NiMoO4 NA/NF (10.9 mF cm−2) (Figure 6c), suggesting a larger surface active area and more exposed active sites [10]. Figure 6d shows that the charge-transfer resistance of the Ni(P, O)x·MoOx NA/NF electrode (3.4 Ω) is smaller than that of the NiMoO4 NA/NF (3.9 Ω), indicating rapid charge transfer. The large electro-active surface area along with the enhanced charge transfer kinetics of the Ni(P, O)x·MoOx NA/NF are believed to be responsible for the associated higher HER catalytic activity.

Table 1.
Comparison of HER performance for Ni(P, O)x·MoOx NA/NF with Mo-based electrocatalysts.
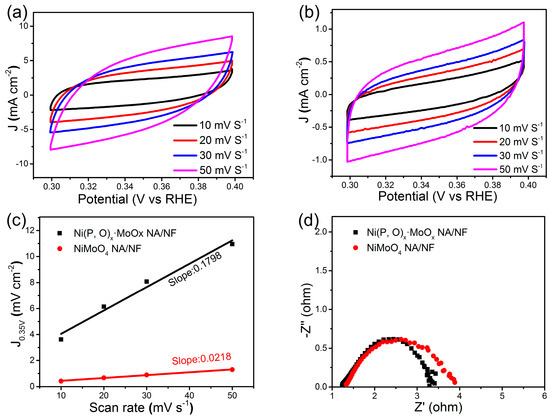
Figure 6.
Cyclic voltammograms of (a) Ni(P, O)x·MoOx NA/NF and (b) NiMoO4 NA/NF; (c) Scan rate-dependent current densities at 0.35 V (vs. reversible hydrogen electrode, RHE); and (d) Nyquist plots of Ni(P, O)x·MoOx NA/NF and NiMoO4 NA/NF.
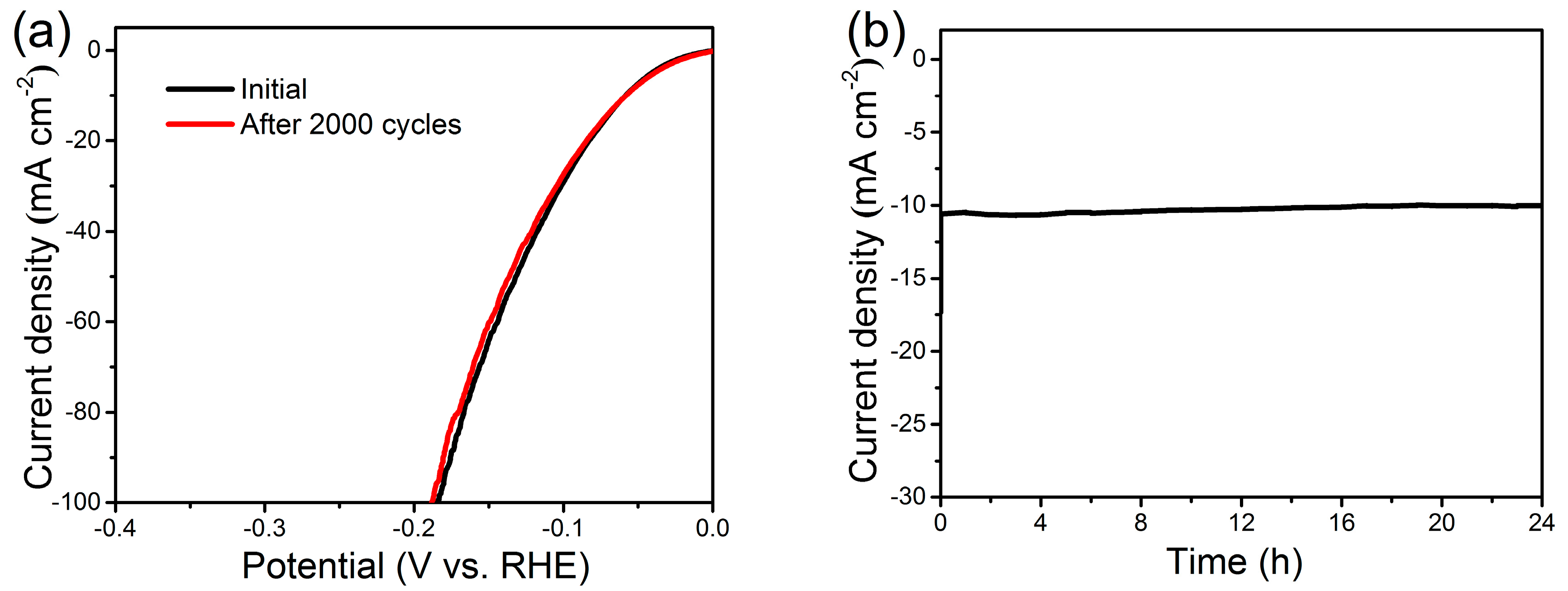
Good catalytic stability is of critical significance for an electrocatalyst when it comes to potentially practical implementation, particularly considering that the HER catalysts work in harsh environments [8]. The Ni(P, O)x·MoOx NA/NF electrocatalyst was first evaluated via a recycling test using LSV method. As shown in Figure 7a, the LSV curves are almost overlapped with a slight loss of the cathodic current densities, indicating a negligible active degradation before and after 2000 scanning cycles. The excellent cycling stability is further validated by the time dependence of the current density curve at a constant overpotential of 60 mV (Figure 7b). The Ni(P, O)x·MoOx NA/NF manifests a stable catalytic current over 24 h, confirming the long-term durability of the electrocatalytic activity.
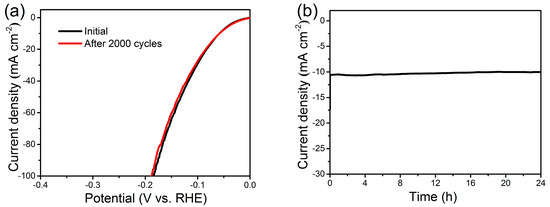
Figure 7.
(a) Polarization curves for Ni(P, O)x·MoOx NA/NF in 1 M KOH initially and after 2000 cycles at a scan rate of 50 mV s−1; (b) Time-dependent current density of Ni(P, O)x·MoOx NA/NF at 60 mV (vs. RHE).
It is believed that the high alkaline HER performance of the Ni(P, O)x·MoOx NA/NF can be attributed to the combination of compositional and geometric advantages: (1) Ni2+ is of great benefit for the adsorption of hydroxyl species, while amorphous MoOx provides catalytically active sites for the adsorption of H* intermediate and further facilitates the subsequent formation of H2. The synergistic cooperation greatly reduces the energy barriers of the initial water decomposition and the subsequent step of H2 generation. (2) Coupling Ni-species and amorphous MoOx generates a strong synergistic effect to significantly improve the stability. (3) The nanowire array offers a larger surface active area with more exposed active sites. (4) The 3D porous and conductive nickel foam not only effectively increases the contact area between active catalyst and electrolyte, but also serves as a robust skeleton to provide strong mechanical adhesion and electric connection to the nanowire array, thereby ensuring facile charge and mass transport, gas bubble release, and good electrode structure for long-term test.
3. Materials and Methods
3.1. Synthesis of Ni(P, O)x·MoOx NA/NF
All chemical reagents were of analytical grade and used as received without further purification. The Ni foam with a thickness of 1.6 mm and dimensions of 2 × 4 cm2 was sonicated in diluted hydrochloric acid (1 M), acetone, deionized water, and ethanol for 10 min, respectively. In a typical synthetic process, 1 mmol Ni(NO3)2·6H2O and 1 mmol Na2MoO4·2H2O were dissolved in 30 mL H2O to form a clear solution. Subsequently, the solution and purified Ni foam were transferred into a 50 mL Teflon-lined stainless autoclave, which was sealed and heated at 160 °C for 6 h in an oven. After the reaction, the resulting light-green Ni foam was rinsed with deionized water and ethanol, then the sample was dried at 60 °C for overnight.
In the next step, the obtained NiMoO4·xH2O NA/NF precursor and 10 mmol NaH2PO2 were placed at two separate positions of the tube furnace with the NaH2PO2 at the upstream side. Subsequently, the samples were heated at 400 °C for 120 min with a ramp rate of 2 °C min−1 under flowing nitrogen. After cooling to room temperature naturally, the resulting electrode was obtained. The mass loading of the as-prepared Ni(P, O)x·MoOx NA/NF on the Ni foam was ~5.4 mg cm−2. The synthesis of NiMoO4 NA/NF was the same as Ni(P, O)x·MoOx NA/NF, just without NaH2PO2.
3.2. Material Characterization
The crystallographic phase of the products was examined by X-ray diffraction (XRD) with Cu Kα radiation (λ = 0.15418 nm) (X’Pert Pro MPD, Philips, Almelo, The Netherlands). The morphology was characterized by field emission scanning electron microscopy (FE-SEM, FEI Nano SEM 450, FEI, Portland, OR, USA) and transmission electron microscopy (TEM, FEI Tecnai F30G2, FEI, Portland, OR, USA). The surface chemistry and elemental analysis of the sample were characterized by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Scientific, Waltham, MA, USA).
3.3. Electrochemical Measurements
The catalytic performances of the electrocatalysts were investigated by using an electrochemical workstation (Solartron 1260 + 1287, Bognor Regis, West Sussex, UK) in a three-electrode system. The Ni(P, O)x·MoOx NA/NF was used as the working electrode; a graphite rod and the saturated calomel electrode (SCE) were used as the counter and reference electrode, respectively. All of the finial potentials were calibrated to a reversible hydrogen electrode (RHE). The polarization curves were corrected with IR compensation. The working electrodes were activated before the measurement by cyclic voltammetric scans with a scan rate of 50 mV s−1. The HER performances of the obtained electrocatalysts were tested from 0.2 to −0.4 V (vs. RHE) in 1 M KOH aqueous solution by LSV with a scanning rate of 2 mV s−1. Electrochemical impedance spectroscopy (EIS) was carried out at −0.2 V (vs. RHE) over a frequency range from 100 kHz to 0.01 Hz with a 10 mV AC dither. To determine the catalytically active surface area of the products, the electrochemical double-layer capacitance (Cdl) of the electrodes was estimated by using CV method in a non-Faradaic range of 0.3–0.4 V (vs. RHE) at various scan rates. A linear relationship between the current densities at 0.35 V (vs. RHE) and scan rate can be plotted to obtain Cdl, the value of which is half of the resulting slope. The catalytically active surface area of different electrocatalysts can be directly compared by the Cdl values, because the Cdl is in proportion to the active surface area [28].
4. Conclusions
In summary, a novel Ni(P, O)x·MoOx nanowire array supported on a Ni foam was prepared via a facile approach. Because of the synergistic effect of the Ni-species and amorphous MoOx, the as-prepared catalyst exhibits excellent electrocatalytic performance in an alkaline media, including a low overpotential of 59 mV at 10 mA cm−2, a small Tafel slope of 54 mV dec−1, and long-term stability. The enhanced electrocatalytic performance demonstrates the advantageous combination of compositional and geometric factors. The present work also provides an avenue to fabricating low-cost alkaline electrocatalysts for practical implementation.
Acknowledgments
The authors acknowledge the financial support by the National Natural Science Foundation of China (51772249, 51402236, 51472204), the Research Fund of the State Key Laboratory of Solidification Processing (NWPU), China (Grant No.: 123-QZ-2015), the Key Laboratory of New Ceramic and Fine Processing and State Key Laboratory of Control and Simulation of Power System and Generation Equipment (Tsinghua University, KF201607, SKLD17KM02), the Fundamental Research Funds for the Central Universities (G2017KY0308) and the Program of Introducing Talents of Discipline to Universities (B08040).
Author Contributions
Jian-Gan Wang and Wei Hua conceived and designed the experiments; Wei Hua and Huanyan Liu performed the experiments; Jian-Gan Wang, Wei Hua and Bingqing Wei analyzed the results and co-wrote this paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Gratzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, G. One-dimensional earth-abundant nanomaterials for water-splitting electrocatalysts. Adv. Sci. 2017, 4, 1600380. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Liu, Q.; Liang, Y.H.; Tian, J.Q.; Asiri, A.M.; Sun, X.P. A cost-effective 3d hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angew. Chem. Int. Ed. 2014, 53, 12855–12859. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlögl, U.; Alshareef, H.N. Plasma-assisted synthesis of NiCoP for efficient overall water splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Zhang, X.; You, T. Defect- and S-rich ultrathin MoS2 nanosheet embedded N-doped carbon nanofibers for efficient hydrogen evolution. J. Mater. Chem. A 2015, 3, 15927–15934. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. Int. Ed. 2014, 53, 9577–9581. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, H.; Li, J.; Yue, X.; Han, Y.; Shen, P.K.; Cui, Y. Porous MoO2 nanosheets as non-noble bifunctional electrocatalysts for overall water splitting. Adv. Mater. 2016, 28, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shen, P.K. Nanoflower-like metallic conductive MoO2 as a high-performance non-precious metal electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 20080–20085. [Google Scholar] [CrossRef]
- Tang, Y.J.; Gao, M.R.; Liu, C.H.; Li, S.L.; Jiang, H.L.; Lan, Y.Q.; Han, M.; Yu, S.H. Porous molybdenum-based hybrid catalysts for highly efficient hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 12928–12932. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Tran, T.V.; Orio, M.; Torelli, S.; Truong, Q.D.; Nayuki, K.; Sasaki, Y.; Chiam, S.Y.; Yi, R.; Honma, I. Coordination polymer structure and revisited hydrogen evolution catalytic mechanism for amorphous molybdenum sulfide. Nat. Mater. 2016, 15, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Liu, H.; Liu, H.; Fu, Z.; Nan, D. Facile synthesis of microsized MnO/C compoistes with high tap density as high performance anodes for Li-ion batteries. Chem. Eng. J. 2017, 328, 591–598. [Google Scholar] [CrossRef]
- Salvatore, D.A.; Dettelbach, K.E.; Hudkins, J.R.; Berlinguette, C.P. Near-infrared-driven decomposition of metal precursor’s yields amorphous electrocatalytic films. Sci. Adv. 2015, 1, e1400215. [Google Scholar] [CrossRef] [PubMed]
- Farrow, C.L.; Bediako, D.K.; Surendranath, Y.; Nocera, D.G.; Billinge, S.J. Intermediate-range structure of self-assembled cobalt-based oxygen-evolving catalyst. J. Am. Chem. Soc. 2013, 135, 6403–6406. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhuo, J.; Du, K.; Chen, B.; Zhu, Z.; Shao, Y.; Li, M. Electrochemically fabricated polypyrrole and MoSx copolymer films as a highly active hydrogen evolution electrocatalyst. Adv. Mater. 2014, 26, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Staszak-Jirkovsky, J.; Malliakas, C.D.; Lopes, P.P.; Danilovic, N.; Kota, S.S.; Chang, K.-C.; Genorio, B.; Strmcnik, D.; Stamenkovic, V.R.; Kanatzidis, M.G.; et al. Design of active and stable Co-Mo-Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2016, 15, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M (Ni, Co, Fe, Mn) hydr(oxy) oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhang, P.; Zhang, H.; Yu, X.; Zhu, J.; Li, Q.; Wang, T. NiMoO4 nanowires supported on Ni foam as novel advanced electrodes for supercapacitors. J. Mater. Chem. A 2013, 1, 9024–9027. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Wu, H.B.; Madhavi, S.; Lou, X.W.D. Controlled growth of NiMoO4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhang, Z.; Zhang, X.; Yin, X.; Li, X.; Liu, X.; Kang, F.; Wei, B. Cation exchange formation of prussian blue analogue submicroboxes for high-performance Na-ion hybrid supercapacitors. Nano Energy 2017, 39, 647–653. [Google Scholar] [CrossRef]
- Du, C.; Shang, M.; Mao, J.; Song, W. Hierarchical MoP/Ni2P heterostructures on nickel foam for efficient water splitting. J. Mater. Chem. A 2017, 5, 15940–15949. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Wang, Y. A new approach to synthesize MoO2@C for high-rate lithium ion batteries. J. Mater. Chem. A 2015, 3, 21314–21320. [Google Scholar] [CrossRef]
- Zhong, D.; Liu, L.; Li, D.; Wei, C.; Wang, Q.; Hao, G.; Zhao, Q.; Li, J. Facile and fast fabrication of iron-phosphate supported on nickel foam as a highly efficient and stable oxygen evolution catalyst. J. Mater. Chem. A 2017, 5, 18627–18633. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Duan, Y.; Gao, M.-R.; Lang, C.-C.; Zheng, Y.-R.; Yu, S.-H. A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 2017, 8, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Sun, Y.; Zhu, C.; Li, C.; Zhang, X.; Chen, Y.-J. Bimetallic Ni-Mo nitride nanotubes as highly active and stable bifunctional electrocatalysts for full water splitting. J. Mater. Chem. A 2017, 5, 13648–13658. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Li, G.D.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with nitrogen-rich nanocarbon leads to efficient hydrogen-evolution electrocatalytic sites. Angew. Chem. Int. Ed. 2015, 54, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Wei, S.; Chen, Z.; Mu, S. Flexible molybdenum phosphide nanosheet array electrodes for hydrogen evolution reaction in a wide pH range. Appl. Catal. B 2016, 196, 193–198. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, W.; Hou, D.; Zhou, K.; Li, G.; Tang, Z.; Li, L.; Chen, S. Porous metallic MoO2-supported MoS2 nanosheets for enhanced electrocatalytic activity in the hydrogen evolution reaction. Nanoscale 2015, 7, 5203–5208. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Sun, Y.; Liu, Y.; Li, J. Flawed MoO2 belts transformed from MoO3 on a graphene template for the hydrogen evolution reaction. Nanoscale 2015, 7, 7040–7044. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Jaramillo, T.F. Molybdenum phosphosulfide: An active, acid-stable, earth-abundant catalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 14433–14437. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).