Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles
Abstract
:1. Introduction
2. Synthesis of NiO Nanoparticles
3. Results and Discussion
3.1. Electron Density Analysis
3.2. EDS Analysis
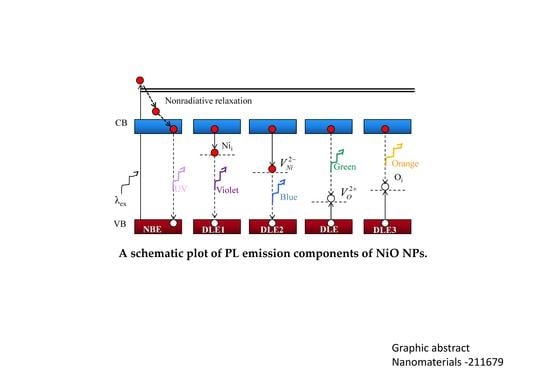
3.3. Photoluminescence
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klimm, D. Electronic materials with a wide band gap: Recent developments. IUCrJ 2014, 1, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.S.; Gandhi, A.C.; Pandit, S.D.; Pant, J.; Chan, T.-S.; Cheng, C.-L.; Ma, Y.-R.; Wu, S.Y. Oxygen induced strained ZnO nanoparticles: An investigation of Raman scattering and visible photoluminescence. J. Mat. Chem. C 2014, 2, 7264–7274. [Google Scholar] [CrossRef]
- Choi, W.S.; Chisholm, M.F.; Singh, D.J.; Choi, T.; Jellison, G.E.; Lee, H.N. Wide bandgap tunability in complex transition metal oxides by site-specific substitution. Nat. Commun. 2012, 3, 689. [Google Scholar] [CrossRef] [PubMed]
- Molaei, R.; Bayati, R.; Narayan, J. Crystallographic characteristics and p-type to n-type transition in epitaxial NiO thin film. Cryst. Growth Des. 2013, 13, 5459–5465. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Kalathil, S.; Lee, J.; Cho, M.H. Band gap engineering of CeO2 nanostructure using an electrochemically active biofilm for visible light applications. RSC Adv. 2014, 4, 16782–16791. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Fujii, M. Evidence for Ti interstitial induced extended visible absorption and near infrared photoluminescence from undoped TiO2 nanoribbons: An in situ photoluminescence study. J. Phys. Chem. C 2013, 117, 23402–23411. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interface 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Watson, G.W. On the possibility of p-type SnO2. J. Mater. Chem. 2012, 22, 25236–25245. [Google Scholar] [CrossRef]
- Ghosh, M.; Raychaudhuri, A.K. Electric field induced reversible control of visible photoluminescence from ZnO nanoparticles. Appl. Phys. Lett. 2011, 98, 153109. [Google Scholar] [CrossRef]
- Saravanakumar, S.; Saravanan, R.; Sasikumar, S. Effect of sintering temperature on the magnetic properties and charge density distribution of nano-NiO. Chem. Pap. 2014, 68, 788–797. [Google Scholar] [CrossRef]
- Madhu, G.; Biju, V. Effect of Ni2+ and O2− vacancies on the electrical and optical properties of nanostructured nickel oxide synthesized through a facile chemical route. Physica E 2014, 60, 200–205. [Google Scholar] [CrossRef]
- Kisan, B.; Shyni, P.C.; Layek, S.; Verma, H.C.; Hesp, D.; Dhanak, V.; Krishnamurthy, S.; Perumal, A. Finite size effects in magnetic and optical properties of antiferromagnetic NiO nanoparticles. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Manikandan, A.; Judith Vijaya, J.; John Kennedy, L. Comparative investigation of NiO nano- and microstructures for structural, optical and magnetic properties. Physica E 2013, 49, 117–123. [Google Scholar] [CrossRef]
- Sokolov, V.I.; Pustovarov, V.A.; Churmanov, V.N.; Ivanov, V.Y.; Gruzdev, N.B.; Sokolov, P.S.; Baranov, A.N.; Moskvin, A.S. Unusual X-ray excited luminescence spectra of NiO suggest self-trapping of the d-d charge-transfer exciton. Phys. Rev. B 2012, 86, 115128. [Google Scholar] [CrossRef]
- Jiang, D.Y.; Qin, J.M.; Wang, X.; Gao, S.; Liang, Q.C.; Zhao, J.X. Optical properties of NiO thin films fabricated by electron beam evaporation. Vacuum 2012, 86, 1083–1086. [Google Scholar] [CrossRef]
- Gondal, M.A.; Saleh, T.A.; Drmosh, Q.A. Synthesis of nickel oxide nanoparticles using pulsed laser ablation in liquids and their optical characterization. Appl. Surf. Sci. 2012, 258, 6982–6986. [Google Scholar] [CrossRef]
- Matsubara, K.; Huang, S.; Iwamoto, M.; Pan, W. Enhanced conductivity and gating effect of p-type Li-doped NiO nanowires. Nanoscale 2014, 6, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Devan, R.S.; Lin, J.-H.; Liou, Y.; Ma, Y.-R. An efficient methodology for measurement of the average electrical properties of single one-dimensional NiO nanorods. Sci. Rep. 2013, 3, 3070. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Devan, R.S.; Lin, J.-H.; Ma, Y.-R.; Patil, P.S.; Liou, Y. Efficient electrochromic properties of high-density and large-area arrays of one-dimensional NiO nanorods. Sol. Energy Mater. Sol. Cells 2013, 112, 91–96. [Google Scholar] [CrossRef]
- Gandhi, A.C.; Pant, J.; Pandit, S.D.; Dalimbkar, S.K.; Chan, T.-S.; Cheng, C.-L.; Ma, Y.-R.; Wu, S.Y. Short-range magnon excitation in NiO nanoparticles. J. Phys. Chem. C 2013, 117, 18666–18674. [Google Scholar] [CrossRef]
- Nandy, S.; Maiti, U.N.; Ghosh, C.K.; Chattopadhyay, K.K. Enhanced p-type conductivity and band gap narrowing in heavily Al doped NiO thin films deposited by. J. Phys. Condens. Matter 2009, 21, 115804. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Saha, B.; Mitra, M.; Chattopadhyay, K.K. Effect of oxygen partial pressure on the electrical and optical properties of highly (200) oriented p-type Ni1−x O films by DC sputtering. J. Mater. Sci. 2007, 42, 5766–5772. [Google Scholar] [CrossRef]
- Børseth, T.M.; Svensson, B.G.; Kuznetsov, A.Y.; Klason, P.; Zhao, Q.X.; Willander, M. Identification of oxygen and zinc vacancy optical signals in ZnO. Appl. Phys. Lett. 2006, 89, 262112. [Google Scholar] [CrossRef]
- Gandhi, A.C.; Pant, J.; Wu, S.Y. Dense inter-particle interactions mediated spontaneous exchange bias in NiO nanoparticles. RSC Adv. 2016, 6, 2079. [Google Scholar] [CrossRef]
- Mandal, S.; Banerjee, S.; Menon, K.S.R. Core-shell model of the vacancy concentration and magnetic behavior for antiferromagnetic nanoparticle. Phys. Rev. B 2009, 80, 214420. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Anspoks, A.; Kalinko, A.; Kalendarev, R.; Kuzmin, A. Atomic structure relaxation in nanocrystalline NiO studied by EXAFS spectroscopy: Role of nickel vacancies. Phys. Rev. B 2012, 86, 174114. [Google Scholar] [CrossRef]
- Gandhi, A.; Huang, C.-Y.; Yang, C.; Chan, T.; Cheng, C.-L.; Ma, Y.-R.; Wu, S. Growth mechanism and magnon excitation in NiO nanowalls. Nanoscale Res. Lett. 2011, 6, 485. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.J.; Lu, S.H.; Wu, Z.L.; Wang, Y.S. Size Effects on Properties of NiO Nanoparticles Grown in Alkalisalts. J. Phys. Chem. C 2012, 116, 26043–26051. [Google Scholar] [CrossRef]
- Ghosh, M.; Biswas, K.; Sundaresan, A.; Rao, C.N.R. MnO and NiO nanoparticles: Synthesis and magnetic properties. J. Mater. Chem. 2006, 16, 106–111. [Google Scholar] [CrossRef]
- Waasmaier, D.; Kirfel, A. New analytical scattering-factor functions for free atoms and ions. Acta Crystallogr. A 1995, 51, 416–431. [Google Scholar] [CrossRef]
- Jones, M.; Engtrakul, C.; Metzger, W.K.; Ellingson, R.J.; Nozik, A.J.; Heben, M.J.; Rumbles, G. Analysis of photoluminescence from solubilized single-walled carbon nanotubes. Phys. Rev. B 2005, 71, 115426. [Google Scholar] [CrossRef]
- Alidoust, N.; Toroker, M.C.; Carter, E.A. Revisiting Photoemission and Inverse Photoemission Spectra of Nickel Oxide from First Principles: Implications for Solar Energy Conversion. J. Phys. Chem. B 2014, 118, 7963–7971. [Google Scholar] [CrossRef] [PubMed]
- Alidoust, N.; Toroker, M.C.; Keith, J.A.; Carter, E.A. Significant Reduction in NiO Band Gap Upon Formation of LixNi1−xO alloys: Applications to Solar Energy Conversion. ChemSusChem 2014, 7, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Andre Venter, J.R.B. Optical and electrical properties of NiO for possible dielectric applications. S. Afr. J. Sci. 2011, 107, 6. [Google Scholar] [CrossRef]
- Hüfner, S. Electronic structure of NiO and related 3d-transition-metal compounds. Adv. Phys. 1994, 43, 183–356. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge Carrier Dynamics at TiO2 Particles: Reactivity of Free and Trapped Holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Tamaki, Y.; Furube, A.; Murai, M.; Hara, K.; Katoh, R.; Tachiya, M. Direct Observation of Reactive Trapped Holes in TiO2 Undergoing Photocatalytic Oxidation of Adsorbed Alcohols: Evaluation of the Reaction Rates and Yields. J. Am. Chem. Soc. 2006, 128, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Saito, T. Intrinsic and defect-related luminescence of NiO. Physica B 2009, 404, 4850–4853. [Google Scholar] [CrossRef]





| Bond Critical Point | Peak Charge Density | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA (°C) | Ni–O | O–O | Ni–O | O–O | ||||||
| Ni | O | O | ||||||||
| d(Å) | ρe(e/Å3) | d(Å) | ρe(e/Å3) | d(Å) | ρe(e/Å3) | d(Å) | ρe(e/Å3) | d(Å) | ρe(e/Å3) | |
| 400 | 1.0914 | 0.4957 | 1.4841 | 0.1728 | 0 | 164.386 | 2.0989 | 18.8958 | 2.9623 | 18.8958 |
| 500 | 1.0910 | 0.5189 | 1.4835 | 0.1766 | 0 | 148.289 | 2.0983 | 16.7023 | 2.9615 | 16.7023 |
| 600 | 1.0906 | 0.5667 | 1.4829 | 0.1912 | 0 | 105.241 | 2.0979 | 15.7964 | 2.9609 | 15.7964 |
| 700 | 1.0900 | 0.5648 | 1.4823 | 0.1939 | 0 | 127.527 | 2.0954 | 15.8163 | 2.9574 | 15.8163 |
| 800 | 1.0896 | 0.5744 | 1.4817 | 0.1939 | 0 | 126.492 | 2.0951 | 15.2787 | 2.9574 | 15.2787 |
| Mean Size (nm) | UV Region (nm) | Visible Region (nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NBE | FWHM | DLE1 | FWHM | DLE2 | FWHM | DLE | FWHM | DLE3 | FWHM | |
| 16.6 ± 0.7 | 357 ± 1 | 35 | 417 ± 1 | 48 | 459 ± 1 | 60 | 523.6 ± 0.3 | 78 | 604 ± 1 | 50 |
| 19.5 ± 0.6 | 351 ± 1 | 36 | 418 ± 1 | 46 | 451 ± 1 | 48 | 518.4 ± 0.3 | 85 | 611 ± 1 | 43 |
| 29 ± 4 | 356 ± 2 | 39 | 420 ± 5 | 57 | 468 ± 4 | 58 | 521 ± 3 | 75 | 598 ± 2 | 59 |
| 31 ± 1 | 335.4 ± 0.1 | 40 | 415 ± 1 | 28 | 448 ± 1 | 36 | 520 ± 1 | 73 | 605 ± 1 | 42 |
| 54 ± 6 | 333.2 ± 0.2 | 48 | 430 ± 2 | 25 | 451.3 ± 0.2 | 16 | 520.2 ± 0.1 | 72 | 595 ± 1 | 55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, A.C.; Wu, S.Y. Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles. Nanomaterials 2017, 7, 231. https://doi.org/10.3390/nano7080231
Gandhi AC, Wu SY. Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles. Nanomaterials. 2017; 7(8):231. https://doi.org/10.3390/nano7080231
Chicago/Turabian StyleGandhi, Ashish Chhaganlal, and Sheng Yun Wu. 2017. "Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles" Nanomaterials 7, no. 8: 231. https://doi.org/10.3390/nano7080231
APA StyleGandhi, A. C., & Wu, S. Y. (2017). Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles. Nanomaterials, 7(8), 231. https://doi.org/10.3390/nano7080231