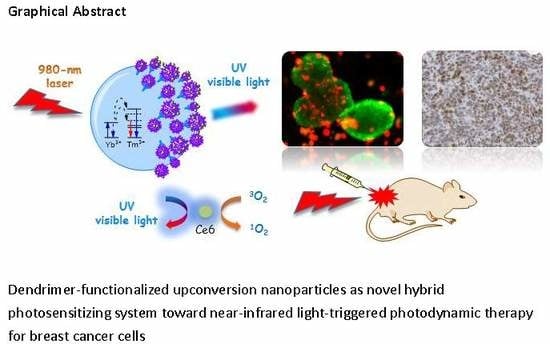
Near-Infrared-Triggered Photodynamic Therapy toward Breast Cancer Cells Using Dendrimer-Functionalized Upconversion Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Lanthanide-Doped UCNPs
2.2. Fabrication of UCNPs through Ligand Exchange and the LBL Strategy
2.3. The Evaluation of Photoactivity and Singlet Oxygen (1O2) Formation
2.4. In Vitro PDT Analysis for Human-Breast Cancer Cells
2.5. In Vivo Assessment for DNA Damage in Tumor Tissues
3. Conclusions
4. Materials and Methods
4.1. Materials and Instruments
4.2. Synthesis of Hybrid UCNPs
4.3. The Singlet Oxygen Assay
4.4. Cell Culture and Tumorsphere Cultivation
4.5. Cell Viability Assay
4.6. In Vivo Assessment for Tumor Tissues
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.-S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, W.Y.-W.; Wu, T.; Xu, J.; Zhang, K.; Wong, D.S.H.; Li, R.; Li, G.; Bian, L. Near-infrared light-triggered release of small molecules for controlled differentiation and long-term tracking of stem cells in vivo using upconversion nanoparticles. Biomaterials 2016, 110, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Zhang, F. Bioapplications and biotechnologies of upconversion nanoparticle-based nanosensors. Analyst 2016, 141, 3601–3620. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiang, J.; Zhao, H.; Liang, H.; Huang, J.; Li, Y.; Pan, J.; Zhou, H.; Zhang, X.; Wang, J.H.; et al. Human amniotic fluid stem cells labeled with up-conversion nanoparticles for imaging-monitored repairing of acute lung injury. Biomaterials 2016, 100, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zheng, X.; Zhang, X.; Yin, W.; Yu, J.; Wang, D.; Zhang, Z.; Yang, X.; Gu, Z.; Zhao, Y. TPGS-stabilized NaYbF4:Er upconversion nanoparticles for dual-modal fluorescent/CT imaging and anticancer drug delivery to overcome multi-drug resistance. Biomaterials 2015, 40, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, A.; Wu, X.; Tokatli-Apollon, A.; El-Rifai, M.; Lee, H.; Zhang, Y.; Wang, C.; Liu, Z.; Chan, E.M.; Duan, C.; et al. Amplifying the Red-Emission of Upconverting Nanoparticles for Biocompatible Clinically Used Prodrug-Induced Photodynamic Therapy. ACS Nano 2014, 8, 10621–10630. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Gu, Z.; Zhou, L.; Yin, W.; Liu, X.; Yan, L.; Jin, S.; Ren, W.; Xing, G.; Li, S.; et al. Mn2+ Dopant-Controlled Synthesis of NaYF4:Yb/Er Upconversion Nanoparticles for In Vivo Imaging and Drug Delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, N.; Liu, Z.; Sha, W.; Chen, D.; Xu, Q.; Lu, J. Amphiphilic copolymer coated upconversion nanoparticles for near-infrared light-triggered dual anticancer treatment. Nanoscale 2014, 6, 14903–14910. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Yang, C.; Shao, W.; Damasco, J.; Wang, X.; Ågren, H.; Prasad, P.; Chen, G. Enhanced Upconversion Luminescence in Yb3+/Tm3+-Codoped Fluoride Active Core/Active Shell/Inert Shell Nanoparticles through Directed Energy Migration. Nanomaterials 2014, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, C.-X.; Chen, J.-T.; Yan, X.-P. A Dual-Targeting Upconversion Nanoplatform for Two-Color Fluorescence Imaging-Guided Photodynamic Therapy. Anal. Chem. 2014, 86, 3263–3267. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Zheng, W.; Zhu, H.; Lu, S.; Ma, E.; Tu, D.; Zhou, S.; Huang, M.; Chen, X. Lanthanide-doped upconversion nanoparticles electrostatically coupled with photosensitizers for near-infrared-triggered photodynamic therapy. Nanoscale 2014, 6, 8274–8282. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Liu, Y.; Wang, X.; Ma, X.; Deng, Z.; Li, Y.; Liu, Z. Imaging-Guided pH-Sensitive Photodynamic Therapy Using Charge Reversible Upconversion Nanoparticles under Near-Infrared Light. Adv. Funct. Mater. 2013, 23, 3077–3086. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, M.; Kong, X.; Zhang, Y.; Zeng, Q.; Sun, Z.; Buma, W.J.; Zhang, H. Separately doped upconversion-C60 nanoplatform for NIR imaging-guided photodynamic therapy of cancer cells. Chem. Commun. 2013, 49, 3224–3226. [Google Scholar] [CrossRef] [PubMed]
- Rapozzi, V.; Jori, G. Resistance to Photodynamic Therapy in Cancer; Springer International Publishing: Cham, Switzerland, 2015; p. 248. [Google Scholar]
- Zhao, L.; Peng, J.; Huang, Q.; Li, C.; Chen, M.; Sun, Y.; Lin, Q.; Zhu, L.; Li, F. Near-Infrared Photoregulated Drug Release in Living Tumor Tissue via Yolk-Shell Upconversion Nanocages. Adv. Funct. Mater. 2014, 24, 363–371. [Google Scholar] [CrossRef]
- Qian, H.S.; Guo, H.C.; Ho, P.C.-L.; Mahendran, R.; Zhang, Y. Mesoporous-Silica-Coated Up-Conversion Fluorescent Nanoparticles for Photodynamic Therapy. Small 2009, 5, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Xu, D. Formation of Yolk/SiO2 Shell Structures Using Surfactant Mixtures as Template. J. Am. Chem. Soc. 2009, 131, 2774–2775. [Google Scholar] [CrossRef] [PubMed]
- Budijono, S.J.; Shan, J.; Yao, N.; Miura, Y.; Hoye, T.; Austin, R.H.; Ju, Y.; Prud’homme, R.K. Synthesis of Stable Block-Copolymer-Protected NaYF4:Yb3+, Er3+ Up-Converting Phosphor Nanoparticles. Chem. Mater. 2010, 22, 311–318. [Google Scholar] [CrossRef]
- Bogdan, N.; Vetrone, F.; Roy, R.; Capobianco, J.A. Carbohydrate-coated lanthanide-doped upconverting nanoparticles for lectin recognition. J. Mater. Chem. 2010, 20, 7543–7550. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Nemati Kharat, A.; Adeli, M. Polyamidoamine and polyglycerol; their linear, dendritic and linear-dendritic architectures as anticancer drug delivery systems. J. Mater. Chem. B 2015, 3, 3896–3921. [Google Scholar] [CrossRef]
- Liu, H.; Shen, M.; Zhao, J.; Zhu, J.; Xiao, T.; Cao, X.; Zhang, G.; Shi, X. Facile formation of folic acid-modified dendrimer-stabilized gold-silver alloy nanoparticles for potential cellular computed tomography imaging applications. Analyst 2013, 138, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Gopinath, P. Chemically Cross-Linked Hybrid Nanogels of Alginate and PAMAM Dendrimers as Efficient Anticancer Drug Delivery Vehicles. ACS Biomater. Sci. Eng. 2016, 2, 213–223. [Google Scholar] [CrossRef]
- Siriviriyanun, A.; Imae, T.; Calderó, G.; Solans, C. Phototherapeutic functionality of biocompatible graphene oxide/dendrimer hybrids. Colloids Surf. B Biointerfaces 2014, 121, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, E.R.; Lin, A.Y.; Yan, J.; Luo, L.; Foster, A.E.; Drezek, R.A. Optimization of PAMAM-gold nanoparticle conjugation for gene therapy. Biomaterials 2014, 35, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Worden, J.G.; Dai, Q.; Huo, Q. A nanoparticle-dendrimer conjugate prepared from a one-step chemical coupling of monofunctional nanoparticles with a dendrimer. Chem. Commun. 2006, 14, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-J.; Hu, C.-C.; Chu, C.-C.; Imae, T. Intrinsically Fluorescent PAMAM Dendrimer as Gene Carrier and Nanoprobe for Nucleic Acids Delivery: Bioimaging and Transfection Study. Biomacromolecules 2011, 12, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Ribes, M.; García-Díaz, M.; Busom, M.; Nonell, S.; Semino, C.E. Toward a 3D Cellular Model for Studying In Vitro the Outcome of Photodynamic Treatments: Accounting for the Effects of Tissue Complexity. Tissue Eng. Part A 2013, 19, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tian, X.-J.; Yang, W.-L.; Ehrenberg, B.; Chen, J.-Y. The conjugates of gold nanorods and chlorin e6 for enhancing the fluorescence detection and photodynamic therapy of cancers. Phys. Chem. Chem. Phys. 2013, 15, 15727–15733. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Dong, Z.; Liu, Y.; Yin, S.; Cheng, L.; Xi, W.; Xiang, J.; Liu, K.; Li, Y.; Liu, Z. Engineering of Multifunctional Nano-Micelles for Combined Photothermal and Photodynamic Therapy Under the Guidance of Multimodal Imaging. Adv. Funct. Mater. 2014, 24, 6492–6502. [Google Scholar] [CrossRef]
- Boyer, J.-C.; Carling, C.-J.; Gates, B.D.; Branda, N.R. Two-Way Photoswitching Using One Type of Near-Infrared Light, Upconverting Nanoparticles, and Changing Only the Light Intensity. J. Am. Chem. Soc. 2010, 132, 15766–15772. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The Active-Core/Active-Shell Approach: A Strategy to Enhance the Upconversion Luminescence in Lanthanide-Doped Nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Zhou, H.-P.; Xu, C.-H.; Sun, W.; Yan, C.-H. Clean and Flexible Modification Strategy for Carboxyl/Aldehyde-Functionalized Upconversion Nanoparticles and Their Optical Applications. Adv. Funct. Mater. 2009, 19, 3892–3900. [Google Scholar] [CrossRef]
- Wang, D.; Chen, C.; Ke, X.; Kang, N.; Shen, Y.; Liu, Y.; Zhou, X.; Wang, H.; Chen, C.; Ren, L. Bioinspired Near-Infrared-Excited Sensing Platform for In Vitro Antioxidant Capacity Assay Based on Upconversion Nanoparticles and a Dopamine-Melanin Hybrid System. ACS Appl. Mater. Interfaces 2015, 7, 3030–3040. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Lu, E.; Pichaandi, J.; Cao, P.; Nitz, M.; Winnik, M.A. Quantification of Surface Ligands on NaYF4 Nanoparticles by Three Independent Analytical Techniques. Chem. Mater. 2015, 27, 4899–4910. [Google Scholar] [CrossRef]
- Chu, C.-C.; Imae, T. Fluorescence Investigations of Oxygen-Doped Simple Amine Compared with Fluorescent PAMAM Dendrimer. Macromol. Rapid Commun. 2009, 30, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.I.; Bae, Y.; Bard, A.J. Strong Blue Photoluminescence and ECL from OH-Terminated PAMAM Dendrimers in the Absence of Gold Nanoparticles. J. Am. Chem. Soc. 2004, 126, 8358–8359. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Imae, T. Fluorescence Emission from Dendrimers and Its pH Dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Z.; Jiang, M.; Que, D.; Shi, S.; Zheng, N. Preparation and photodynamic therapy application of NaYF4:Yb, Tm-NaYF4:Yb, Er multifunctional upconverting nanoparticles. New J. Chem. 2013, 37, 1782–1788. [Google Scholar] [CrossRef]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.Q.; Teng, C.P.; Ye, E.; Loh, X.J. Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int. J. Nanomed. 2015, 10, 419–432. [Google Scholar]
- Lee, C.-H.; Yu, C.-C.; Wang, B.-Y.; Chang, W.-W. Tumorsphere as an effective in vitro platform for screening anti-cancer stem cell drugs. Oncotarget 2015, 7, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-Y.; Fong, P.-C.; Yu, C.-C.; Tsai, W.-C.; Tzeng, Y.-M.; Chang, W.-W. Methyl Antcinate A Suppresses the Population of Cancer Stem-Like Cells in MCF7 Human Breast Cancer Cell Line. Molecules 2013, 18, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lou, X.; Zhang, Z.; Ingram, P.; Yoon, E. High-Throughput Cancer Cell Sphere Formation for Characterizing the Efficacy of Photo Dynamic Therapy in 3D Cell Cultures. Sci. Rep. 2015, 5, 12175. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. DNA Base Damage by Reactive Oxygen Species, Oxidizing Agents, and UV Radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; François, M.; Fenech, M.F.; Leifert, W.R. Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat. Res. Rev. Mutat. Res. 2015, 766, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Kuan, Y.-D.; Chen, M.-C.; Lin, S.-T.; Lee, C.-H. Tracking of mouse breast cancer stem-like cells with Salmonella. Exp. Biol. Med. 2012, 237, 1189–1196. [Google Scholar] [CrossRef] [PubMed]












© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-Y.; Liao, M.-L.; Hong, G.-C.; Chang, W.-W.; Chu, C.-C. Near-Infrared-Triggered Photodynamic Therapy toward Breast Cancer Cells Using Dendrimer-Functionalized Upconversion Nanoparticles. Nanomaterials 2017, 7, 269. https://doi.org/10.3390/nano7090269
Wang B-Y, Liao M-L, Hong G-C, Chang W-W, Chu C-C. Near-Infrared-Triggered Photodynamic Therapy toward Breast Cancer Cells Using Dendrimer-Functionalized Upconversion Nanoparticles. Nanomaterials. 2017; 7(9):269. https://doi.org/10.3390/nano7090269
Chicago/Turabian StyleWang, Bing-Yen, Ming-Liang Liao, Guan-Ci Hong, Wen-Wei Chang, and Chih-Chien Chu. 2017. "Near-Infrared-Triggered Photodynamic Therapy toward Breast Cancer Cells Using Dendrimer-Functionalized Upconversion Nanoparticles" Nanomaterials 7, no. 9: 269. https://doi.org/10.3390/nano7090269
APA StyleWang, B.-Y., Liao, M.-L., Hong, G.-C., Chang, W.-W., & Chu, C.-C. (2017). Near-Infrared-Triggered Photodynamic Therapy toward Breast Cancer Cells Using Dendrimer-Functionalized Upconversion Nanoparticles. Nanomaterials, 7(9), 269. https://doi.org/10.3390/nano7090269