Synthesis and Characterization of Rh/B–TNTs as a Recyclable Catalyst for Hydroformylation of Olefin Containing –CN Functional Group
Abstract
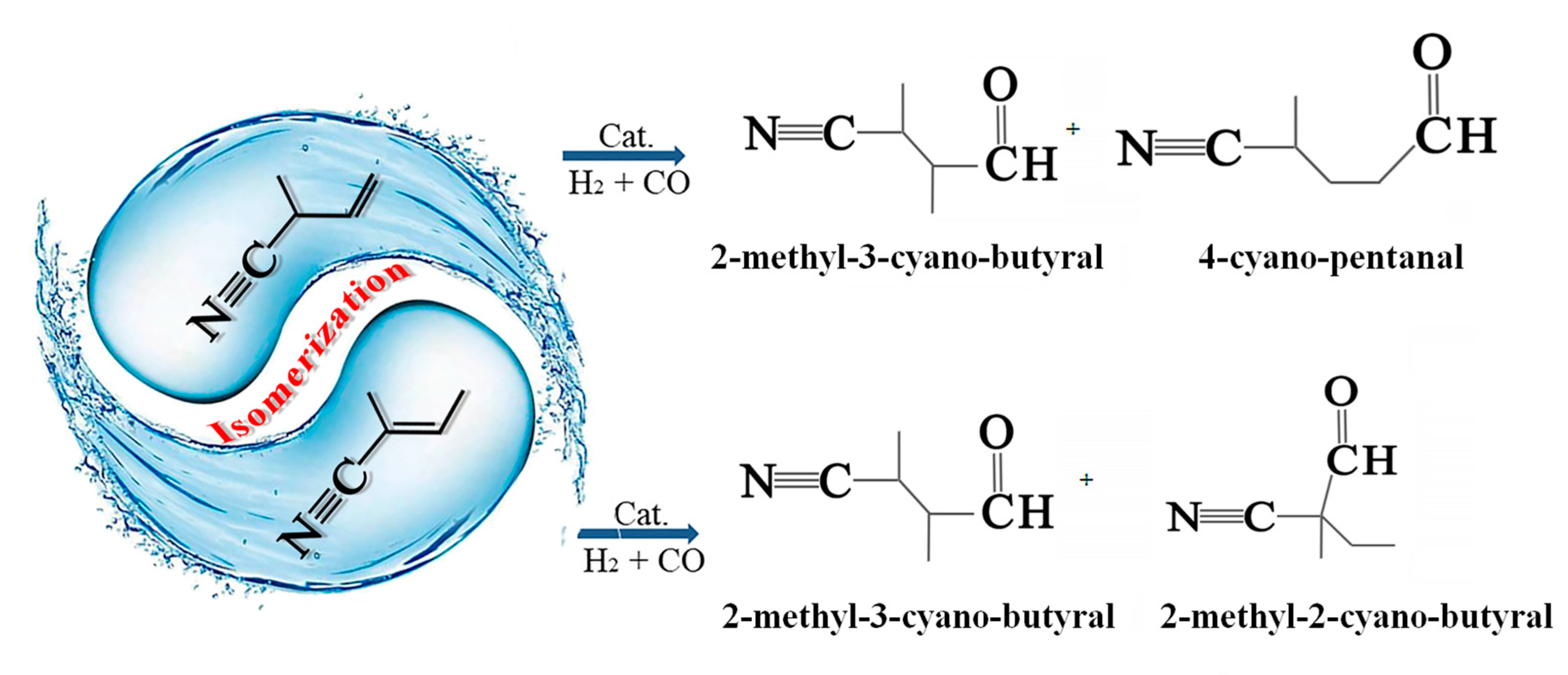
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Catalysts
2.2. Synthesis of Rh/TNTs and Rh/B–TNTs
2.3. Evaluation of Catalytic Performance of Catalysts for Hydroformylation
2.4. Characterization
3. Results and Discussion
3.1. BET and ICP Analysis
3.2. XRD Analysis
3.3. SEM Analysis
3.4. TEM Analysis
3.5. XPS Analysis
3.6. NH3-TPD Analysis
3.7. Catalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piras, I.; Jennerjahn, R.; Jackstell, R.; Spannenberg, A.; Franke, R.; Beller, M. A General and Efficient Iridium-Catalyzed Hydroformylation of Olefins. Angew. Chem. 2011, 50, 280–284. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Wei, B.; Li, X.X.; Yang, Y.S.; Liu, Y.; Lv, H.; Zhang, X.M. Rhodium-catalyzed desymmetrization by hydroformylation of cyclopentenes: synthesis of chiral carbocyclic nucleosides. Angew. Chem. Int. Ed. 2016, 55, 6511–6514. [Google Scholar] [CrossRef] [PubMed]
- Dydio, P.; Reek, J.N.H. Supramolecular control of selectivity in hydroformylation of vinyl arenes: easy access to valuable beta-aldehyde intermediates. Angew. Chem. Int. Ed. 2013, 52, 3878–3882. [Google Scholar] [CrossRef] [PubMed]
- Pospech, J.; Fleischer, I.; Franke, R.; Buchholz, S.; Beller, M. Alternative metals for homogeneous catalyzed hydroformylation reactions. Angew. Chem. Int. Ed. 2013, 52, 2852–2872. [Google Scholar] [CrossRef] [PubMed]
- Neves, Â.C.B.; Calvete, M.J.F.; Pinho e Melo, T.M.V.D.; Pereira, M.M. Immobilized catalysts for hydroformylation reactions: A versatile tool for aldehyde synthesis. Eur. J. Org. Chem. 2012, 32, 6309. [Google Scholar] [CrossRef]
- Paganelli, S.; Piccolo, O.; Baldi, F.; Gallo, M.; Tassini, R.; Rancan, M.; Armelao, L. A new biogenerated Rh-based catalyst for aqueous biphasic hydroformylation. Catal. Commun. 2015, 71, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Dai, Z.F.; Liu, X.L.; Sheng, N.; Deng, F.; Meng, X.J.; Xiao, F.S. Highly Efficient Heterogeneous Hydroformylation over Rh-Metalated Porous Organic Polymers: Synergistic Effect of High Ligand Concentration and Flexible Framework. J. Am. Chem. Soc. 2015, 137, 5204–5209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Yan, L.; Li, C.Y.; Jiang, M.; Wang, W.L.; Ding, Y.J. Highly efficient porous organic copolymer supported Rh catalysts for heterogeneous hydroformylation of butenes. Appl. Catal. A Gen. 2018, 551, 98–105. [Google Scholar] [CrossRef]
- Garcia, M.A.S.; Oliveira, K.C.B.; Costa, J.C.S.; Corio, P.; Gusevskaya, E.V.; Santos, E.N.D.; Rossi, L.M. Rhodium Nanoparticles as Precursors for the Preparation of an Efficient and Recyclable Hydroformylation Catalyst. Chem. Cat. Chem. 2015, 7, 1566–1572. [Google Scholar] [CrossRef]
- Khokhar, M.D.; Shukla, R.S.; Jasra, R.V. Hydroformylation of dihydrofurans catalyzed by rhodium complex encapsulated hexagonal mesoporous silica. J. Mol. Catal. A Chem. 2015, 400, 1–6. [Google Scholar] [CrossRef]
- Ma, Y.B.; Fu, J.; Gao, Z.X.; Zhang, L.B.; Li, C.Y.; Wang, T.F. Dicyclopentadiene Hydroformylation to Value-Added Fine Chemicals over Magnetically Separable Fe3O4-Supported Co-Rh Bimetallic Catalysts: Effects of Cobalt Loading. Catalysts 2017, 7, 103. [Google Scholar] [CrossRef]
- Chikkali, S.H.; Bellini, R.; De Bruin, B.D.; Vlugt, J.I.V.D.; Reek, J.N.H. Highly selective asymmetric Rh-catalyzed hydroformylation of heterocyclic olefins. J. Am. Chem. Soc. 2012, 134, 6607–6616. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ganesh, V.; Sakthivel, A. Rhodium incorporated monometallic cobalt hydrotalcite-type materials: Preparation and its applications for the hydroformylation of alkenes. Appl. Catal. A 2018, 555, 155–160. [Google Scholar] [CrossRef]
- Kontkanen, M.-L.; Tuikka, M.; Kinnunen, N.M.; Suvanto, S.; Haukka, M. Hydroformylation of 1-Hexene over Rh/Nano-Oxide Catalysts. Catalysts 2013, 3, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.K.; Hu, X.J.; Zhu, B.L.; Wang, S.R.; Zhang, S.M.; Huang, W.P. Synthesis and characterization of TiO2 nanotube supported Rh-nanoparticle catalysts for regioselective hydroformylation of vinyl acetate. RSC Adv. 2014, 4, 62215–62222. [Google Scholar] [CrossRef]
- Hu, X.J.; Shi, Y.K.; Zhang, YJ.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Nanotubular TiO2-supported amorphous Co–B catalysts and their catalytic performances for hydroformylation of cyclohexene. Catal. Commun. 2015, 59, 45–49. [Google Scholar] [CrossRef]
- Zhou, G.B.; Pei, Y.; Jiang, Z.; Fan, K.N.; Qiao, M.H.; Sun, B.; Zong, B.N. Doping effects of B in ZrO2 on structural and catalytic properties of Ru/B-ZrO2 catalysts for benzene partial hydrogenation. J. Catal. 2014, 311, 393–403. [Google Scholar] [CrossRef]
- Urbano, F.J.; Aramendía, M.A.; Marinas, A.; Marinas, J.M. An insight into the Meerwein–Ponndorf–Verley reduction of α,β-unsaturated carbonyl compounds: Tuning the acid–base properties of modified zirconia catalysts. J. Catal. 2009, 268, 79–88. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Suzuki, T.; Suzuki, A.; Kawanami, H. In situ synthesized Pd nanoparticles supported on B-MCM-41: an efficient catalyst for hydrogenation of nitroaromatics in supercritical carbon dioxide. Green Chem. 2012, 14, 3415. [Google Scholar] [CrossRef]
- Yin, A.Y.; Qu, J.W.; Guo, X.Y.; Dai, W.L.; Fan, K.N. The influence of B-doping on the catalytic performance of Cu/HMS catalyst for the hydrogenation of dimethyloxalate. Appl. Catal. A 2011, 400, 39–47. [Google Scholar] [CrossRef]
- Shi, Y.K.; Hu, X.J.; Chen, L.; Lu, Y.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Boron modified TiO2 nanotubes supported Rh-nanoparticle catalysts for highly efficient hydroformylation of styrene. New J. Chem. 2017, 41, 6120. [Google Scholar] [CrossRef]
- You, C.; Li, X.X.; Yang, Y.H.; Yang, Y.S.; Tan, X.F.; Li, S.L.; Wei, B.; Lv, H.; Chung, L.W.; Zhang, X.M. Silicon-oriented regio- and enantioselective rhodium-catalyzed hydroformylation. Nat. Commun. 2018, 9, 2045. [Google Scholar] [CrossRef] [PubMed]
- Chuai, H.Y.; Liu, X.T.; Chen, Y.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. Hydroformylation of vinyl acetate and cyclohexene over TiO2 nanotube supported Rh and Ru nanoparticle catalysts. RSC Adv. 2018, 8, 12053–12059. [Google Scholar] [CrossRef]
- Clark, T.P.; Landis, C.R.; Freed, S.L.; Klosin, J.; Abboud, K.A. Highly Active, Regioselective, and Enantioselective Hydroformylation with Rh Catalysts Ligated by Bis-3,4-diazaphospholanes. J. Am. Chem. Soc. 2005, 127, 5040–5042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelsen, E.R.; Brezny, A.C.; Landis, C.R. Interception and Characterization of Catalyst Species in Rhodium Bis(diazaphospholane)-Catalyzed Hydroformylation of Octene, Vinyl Acetate, Allyl Cyanide, and 1-Phenyl-1,3-butadiene. J. Am. Chem. Soc. 2015, 137, 14208–14219. [Google Scholar] [CrossRef] [PubMed]
- Le Goanvic, L.; Ternel, J.; Couturier, J.-L.; Dubois, J.-L.; Carpentier, J.-F. Rhodium-Biphephos-Catalyzed Tandem Isomerization–Hydroformylation of Oleonitrile. Catalysts 2018, 8, 21. [Google Scholar] [CrossRef]
- An, H.Q.; Li, J.X.; Zhou, J.; Li, K.R.; Zhu, B.L.; Huang, W.P. Iron-coated TiO2 nanotubes and their photocatalytic performance. J. Mater. Chem. 2010, 20, 603–610. [Google Scholar] [CrossRef]
- In, S.; Orlov, A.; Berg, R.; García, F.; Pedrosa-Jimenez, S.; Tikhov, M.S.; Wright, D.S.; Lambert, R.M. Effective Visible Light-Activated B-Doped and B,N-Codoped TiO2 Photocatalysts. J. Am. Chem. Soc. 2007, 129, 13790–13791. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, D.H.; Rey, A.; Álvarez, P.M.; Beltrán, F.J.; Li Puma, G. Boron doped TiO2 catalysts for photocatalytic ozonation of aqueous mixtures of common pesticides: Diuron, o-phenylphenol, MCPA and terbuthylazine. Appl. Catal. B Environ. 2015, 178, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Berto, T.F.; Sanwald, K.E.; Eisenreich, W.; Gutiérrez, O.Y.; Lercher, J.A. Photoreforming of ethylene glycol over Rh/TiO2 and Rh/GaN:ZnO. J. Catal. 2016, 338, 68–81. [Google Scholar] [CrossRef]
- László, B.; Baán, K.; Oszkó, A.; Erdőhelyi, A.; Kiss, J.; Kónya, Z. Hydrogen evolution in the photocatalytic reaction between methane and water in the presence of CO2 on titanate and titania supported Rh and Au catalysts. Top. Catal. 2018, 61, 875–888. [Google Scholar] [CrossRef]
- László, B.; Baán, K.; Varga, E.; Oszkó, A.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Photo-induced reactions in the CO2-methane system on titanate nanotubes modified with Au and Rh nanoparticles. Appl. Catal. B Environ. 2016, 199, 473–484. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, H.R.; Zhao, Y.J.; Wang, B.; Geng, Y.C.; Lv, J.; Wang, S.P.; Gong, J.L.; Ma, X.B. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant. J. Catal. 2013, 297, 142–150. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, W.H.; Chen, C.C.; Zhao, J.C.; Shuai, Z.G. Efficient Degradation of Toxic Organic Pollutants with Ni2O3/TiO2-xBxunder Visible Irradiation. J. Am. Chem. Soc. 2004, 126, 4782–4783. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Quan, X.; Li, J.Y.; Chen, S.; Yu, H.T.; Chen, G.H. Fabrication of Boron-Doped TiO2 Nanotube Array Electrode and Investigation of Its Photoelectrochemical Capability. J. Phys. Chem. C 2007, 111, 11836–11842. [Google Scholar] [CrossRef]
- Chen, D.M.; Yang, D.; Wang, Q.; Jiang, Z.Y. Effects of Boron Doping on Photocatalytic Activity and Microstructure of Titanium Dioxide Nanoparticles. Ind. Eng. Chem. Res. 2006, 45, 4110–4116. [Google Scholar] [CrossRef]
- Atashbar, M.Z.; Sun, H.T.; Gong, B.; Wlodarski, W.; Lamb, R. XPS study of Nb-doped oxygen sensing TiO2 thin films prepared by sol-gel method. Thin Solid Films 1998, 326, 238–244. [Google Scholar] [CrossRef]
- Pótári, G.; Madarász, D.; Nagy, L.; László, B.; Sápi, A.; Oszkó, A.; Kukovecz, A.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Rh-induced support transformation phenomena in titanate nanowire and nanotube catalysts. Langmuir 2013, 29, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Shriver, D.F. Some relationships between metal cluster chemistry and heterogeneous catalysis. J. Clust. Sci. 1992, 3, 459–467. [Google Scholar] [CrossRef]
- Fu, Z.; Yin, D.; Li, Q.; Zhang, L.; Zhang, Y. Synthesis, characterization and catalytic properties of titanium and boron co-substituted silicalite zeolites. Micropor. Mesopor. Mater. 1999, 29, 351–359. [Google Scholar] [CrossRef]
- Shi, Y.K.; Hu, X.J.; Zhu, B.L.; Wang, S.R.; Zhang, S.M.; Huang, W.P. Hydroformylation of 1-octene over nanotubular TiO2-supported amorphous Co-B catalysts. Chem. Res. Chin. Univ. 2015, 31, 851–857. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.W.; Tan, K.F.; Borgna, A.; Saeys, M. Effect of boron on the stability of Ni catalysts during steam methane reforming. J. Catal. 2009, 261, 158–165. [Google Scholar] [CrossRef]
- Tan, K.F.; Chang, J.; Borgna, A.; Saeys, M. Effect of boron promotion on the stability of cobalt Fischer–Tropsch catalysts. J. Catal. 2011, 280, 50–59. [Google Scholar] [CrossRef]






| Entry | Catalyst | SSA (m2/g) | Rh Content (wt.%) | B Content (wt.%) |
|---|---|---|---|---|
| 1 | TNTs(a) | 227.6 | - | - |
| 2 | Rh/TNTs(a1) | 198.6 | 0.13 | - |
| 3 | B–TNTs(b) | 238.6 | - | 0.56 |
| 4 | Rh/B–TNTs(b1) | 225.2 | 0.16 | 0.56 |
| 5 | B–TNTs (c) | 275.8 | 0 | 0.99 |
| 6 | Rh/B–TNTs(c1) | 268.6 | 0.16 | 0.99 |
| 7 | Rh/B–TNTs(c2) | 270.2 | 0.09 | 0.99 |
| 8 | Rh/B–TNTs(c3) | 267.4 | 0.19 | 0.99 |
| Catalyst | Content of B (wt.%) | Conversion (%) | Isomerism d (%) | TOF b (h−1) | Aldehyde (%) | b:l c |
|---|---|---|---|---|---|---|
| Rh/TNTs(a1) | 0 | 100 | 25.8 | 2663 | 72 | 32:68 |
| Rh/BTNTs(b1) | 0.56 | 100 | 20.4 | 2532 | 79 | 33:67 |
| Rh/BTNTs(c1) | 0.99 | 100 | 17.9 | 3576 | 81 | 31:69 |
| Catalyst | Rh Content (wt.%) | Conversion (%) | Isomerism d (%) | TOF b (h-1) | Aldehyde (%) | b:l c |
|---|---|---|---|---|---|---|
| Rh/B–TNTs(c1) | 0.16 (6.2 × 10−3 mmol) | 100 | 17.9 | 3576 | 81 | 31:69 |
| Rh/B–TNTs(c2) | 0.09 (3.5 × 10−3 mmol) | 86 | 19.1 | 2742 | 68 | 32:68 |
| Rh/B–TNTs(c3) | 0.19 (7.3 × 10−3 mmol) | 100 | 18.2 | 3639 | 79 | 42:58 |
| Catalyst | Temperature (°C) | Conversion (%) | Isomerism d (%) | TOF b (h−1) | Aldehyde (%) | b:l c |
|---|---|---|---|---|---|---|
| Rh/TNTs (a1) | 80 | 66 | 19.5 | 1833 | 46 | 65:35 |
| Rh/TNTs (a1) | 100 | 100 | 20.4 | 3152 | 73 | 39:61 |
| Rh/TNTs (a1) | 120 | 100 | 25.9 | 3263 | 72 | 32:68 |
| Rh/B–TNTs (c1) | 80 | 74 | 13.1 | 1892 | 59 | 62:38 |
| Rh/B–TNTs (c1) | 100 | 100 | 16.8 | 3537 | 81 | 41:59 |
| Rh/B–TNTs (c1) | 120 | 100 | 17.9 | 3576 | 81 | 31:69 |
| Catalyst | Cycle Times | Conversion (%) | Isomerism d (%) | TOF b (h−1) | Aldehyde (%) | b:l c |
|---|---|---|---|---|---|---|
| Rh/B–TNTs(c1) | 1 | 100 | 17.9 | 3576 | 81 | 31:69 |
| Rh/B–TNTs(c1) | 2 | 100 | 20.3 | 3312 | 77 | 29:71 |
| Rh/B–TNTs(c1) | 3 | 92 | 18.8 | 2556 | 80 | 31:69 |
| Rh/B–TNTs(c1) | 4 | 90 | 29.1 | 2202 | 68 | 38:62 |
| Rh/TNTs(a1) | 1 | 100 | 25.9 | 3263 | 72 | 32:68 |
| Rh/TNTs(a1) | 2 | 50 | 28.6 | 1665 | 69 | 35:65 |
| Rh/TNTs(a1) | 3 | trace | 30.5 | - | trace | - |
| Catalyst | Cycle Times | Rh (PPM) |
|---|---|---|
| Rh/B–TNTs(c1) | 1 | 18.8 |
| Rh/B–TNTs(c1) | 2 | 15.7 |
| Rh/B–TNTs(c1) | 3 | 13.0 |
| Rh/B–TNTs(c1) | 4 | 11.2 |
| Rh/TNTs(a1) | 1 | 42.8 |
| Rh/TNTs(a1) | 2 | 28.7 |
| Rh/TNTs(a1) | 3 | 8.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.; Liu, X.; Chen, Y.; Liu, H.; Zhu, B.; Zhang, S.; Huang, W. Synthesis and Characterization of Rh/B–TNTs as a Recyclable Catalyst for Hydroformylation of Olefin Containing –CN Functional Group. Nanomaterials 2018, 8, 755. https://doi.org/10.3390/nano8100755
Su P, Liu X, Chen Y, Liu H, Zhu B, Zhang S, Huang W. Synthesis and Characterization of Rh/B–TNTs as a Recyclable Catalyst for Hydroformylation of Olefin Containing –CN Functional Group. Nanomaterials. 2018; 8(10):755. https://doi.org/10.3390/nano8100755
Chicago/Turabian StyleSu, Penghe, Xiaotong Liu, Ya Chen, Hongchi Liu, Baolin Zhu, Shoumin Zhang, and Weiping Huang. 2018. "Synthesis and Characterization of Rh/B–TNTs as a Recyclable Catalyst for Hydroformylation of Olefin Containing –CN Functional Group" Nanomaterials 8, no. 10: 755. https://doi.org/10.3390/nano8100755
APA StyleSu, P., Liu, X., Chen, Y., Liu, H., Zhu, B., Zhang, S., & Huang, W. (2018). Synthesis and Characterization of Rh/B–TNTs as a Recyclable Catalyst for Hydroformylation of Olefin Containing –CN Functional Group. Nanomaterials, 8(10), 755. https://doi.org/10.3390/nano8100755




