Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
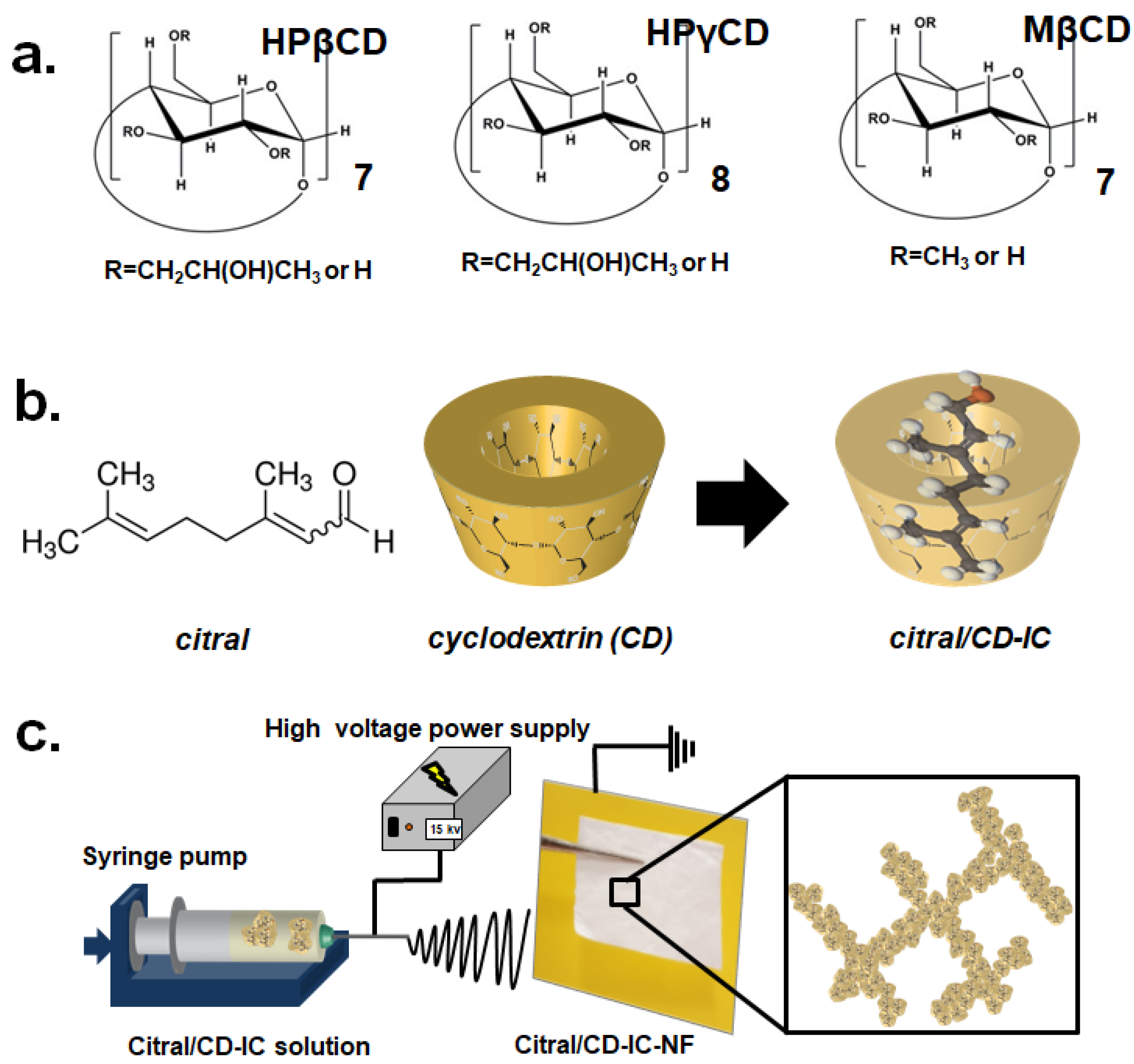
2.2. Preparation of the Citral/CD-IC
2.3. Electrospinning
2.4. Measurements and Characterization
3. Results and Discussion
3.1. Phase Solubility Studies
3.2. Morphology Analyses of Nanofibers
3.3. The Molar Ratio of Citral/CD-IC
3.4. Thermal Analysis of Nanofibers
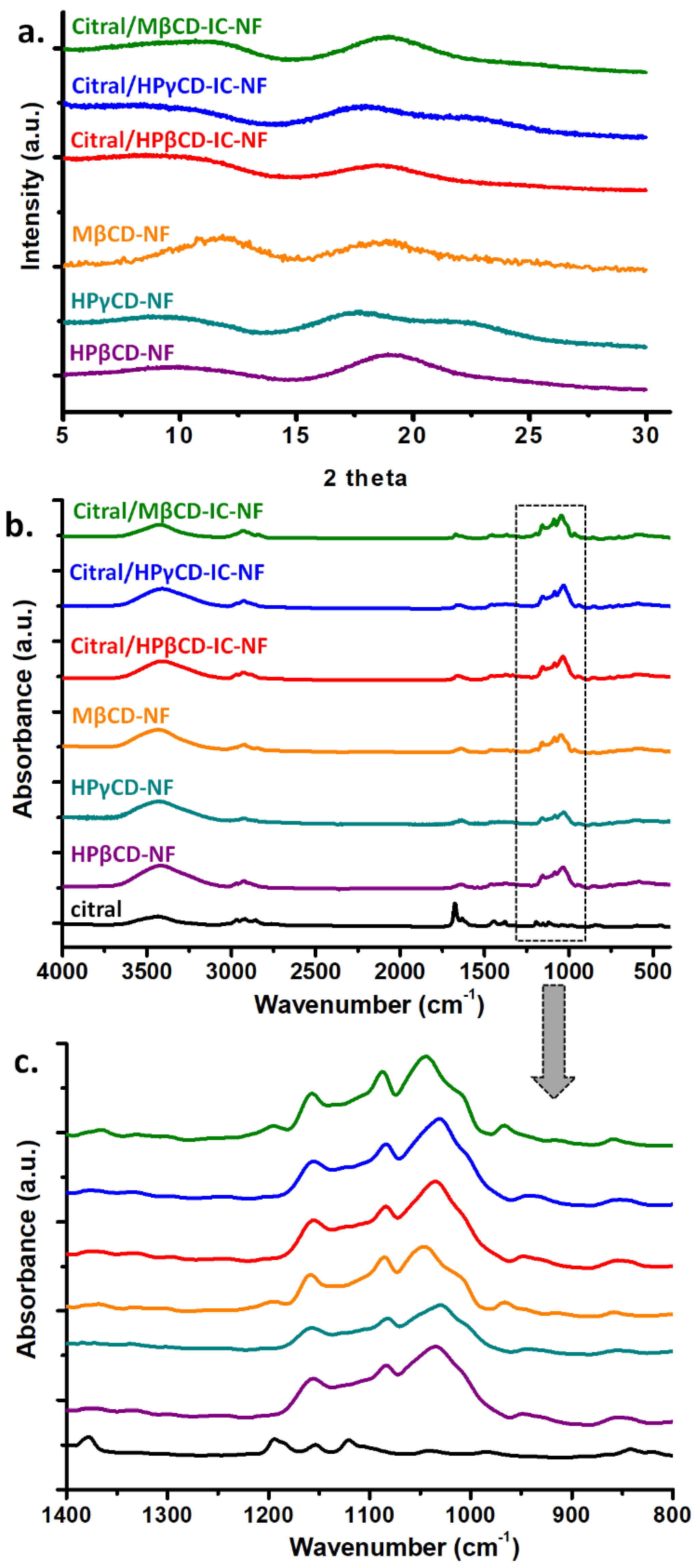
3.5. Structural Characterization of Nanofibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phunpee, S.; Ruktanonchai, U.R.; Yoshii, H.; Assabumrungrat, S.; Soottitantawat, A. Encapsulation of lemongrass oil with cyclodextrins by spray drying and its controlled release characteristics. Biosci. Biotech. Bioch. 2017, 81, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; Wiley: Hoboken, NJ, USA, 2011; pp. 287–295. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and their uses: A. review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Cyclodextrin nanofibers by electrospinning. Chem. Commun. 2010, 46, 6903–6905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 2012, 4, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, M.; Zha, B.B.; Diao, G.W. Correlation of polymer-like solution behaviors with electrospun fiber formation of hydroxypropyl-beta-cyclodextrin and the adsorption study on the fiber. Phys. Chem. Chem. Phys. 2012, 14, 9729–9737. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial electrospun nanofibers from triclosan/cyclodextrin inclusion complexes. Colloid Surf. B 2014, 116, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, A.; Uyar, T. Electrospinning of polymer-free nanofibers from cyclodextrin inclusion complexes. Langmuir 2011, 27, 6218–6226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Keskin, N.O.S.; Tekinay, T.; Uyar, T. Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: Enhanced shelf-life of geraniol with antibacterial and antioxidant properties. Rsc Adv. 2016, 6, 46089–46099. [Google Scholar] [CrossRef]
- Celebioglu, A.; Kayaci-Senirmak, F.; Ipek, S.; Durgun, E.; Uyar, T. Polymer-free nanofibers from vanillin/cyclodextrin inclusion complexes: High thermal stability, enhanced solubility and antioxidant property. Food Funct. 2016, 7, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Keskin, N.O.S.; Kusku, S.I.; Durgun, E.; Tekinay, T.; Uyar, T. Fast-dissolving, prolonged release, and antibacterial cyclodextrin/limonene-inclusion complex nanofibrous webs via polymer-free electrospinning. J. Agr. Food Chem. 2016, 64, 7325–7334. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of cyclodextrin/linalool-inclusion complex nanofibers: Fast-dissolving nanofibrous web with prolonged release and antibacterial activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Electrospun nanofibers from cyclodextrin inclusion complexes with cineole and p-cymene: Enhanced water solubility and thermal stability. Int. J. Food Sci. Tech. 2018, 53, 112–120. [Google Scholar] [CrossRef]
- Celebioglu, A.; Aytac, Z.; Kilic, M.E.; Durgun, E.; Uyar, T. Encapsulation of camphor in cyclodextrin inclusion complex nanofibers via polymer-free electrospinning: Enhanced water solubility, high temperature stability, and slow release of camphor. J. Mater. Sci. 2018, 53, 5436–5449. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Antioxidant vitamin E/cyclodextrin inclusion complex electrospun nanofibers: Enhanced water solubility, prolonged shelf life, and photostability of vitamin E. J. Agr. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Uyar, T.; Kny, E. Electrospun Materials for Tissue Engineering and Biomedical Applications: Research, Design and Commercialization; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Electrospinning: Materials, Processing, and Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Alborzi, S.; Lim, L.T.; Kakuda, Y. Encapsulation of folic acid and its stability in sodium alginate-pectin-poly(ethylene oxide) electrospun fibres. J. Microencapsul. 2013, 30, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Dogan, S.Y.; Tekinay, T.; Uyar, T. Release and antibacterial activity of allyl isothiocyanate/beta-cyclodextrin complex encapsulated in electrospun nanofibers. Colloid Surf. B 2014, 120, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.Y.; Feng, N.N.; Xiao, Z.B.; Zhou, R.J.; Niu, Y.W. Production and pyrolysis characteristics of citral-monochlorotriazinyl-beta-cyclodextrin inclusion complex. J. Therm. Anal. Calorim. 2015, 120, 1811–1817. [Google Scholar] [CrossRef]
- Ruktanonchai, U.R.; Srinuanchai, W.; Saesoo, S.; Sramala, I.; Puttipipatkhachorn, S.; Soottitantawat, A. Encapsulation of citral isomers in extracted lemongrass oil with cyclodextrins: Molecular modeling and physicochemical characterizations. Biosci. Biotech. Bioch. 2011, 75, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Ajisaka, N.; Hara, K.; Mikuni, K.; Hara, K.; Hashimoto, H. Effects of branched cyclodextrins on the solubility and stability of terpenes. Biosci. Biotech. Bioch. 2000, 64, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharmaceut. Biomed. 2008, 47, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Semma, M.; Ichikawa, A. Physicochemical and biological properties of 6(1),6(3) 6(5)-tri-o-alpha-maltosyl-cyclomaltoheptaose (6(1),6(3),6(5)-tri-o-alpha-maltosyl-beta-cyclodextrin). Carbohyd. Res. 2007, 342, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.; de Brauer, C.; Germain, P. Solubilization of the neutral and charged forms of 2,4,6-trichlorophenol by beta-cyclodextrin, methyl-beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin in water. J. Hazard. Mater. 2003, 100, 109–116. [Google Scholar] [CrossRef]
- Shao, H.; Fang, J.; Wang, H.; Lin, T. Effect of electrospinning parameters and polymer concentrations on mechanical-to-electrical energy conversion of randomly-oriented electrospun poly(vinylidene fluoride) nanofiber mats. RSC Adv. 2015, 5, 14345–14350. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Easteal, A.J.; Nikolaidis, M.G.; Quek, S.Y. Influence of solution and processing parameters towards the fabrication of electrospun zein fibers with sub-micron diameter. J. Food Eng. 2012, 109, 645–651. [Google Scholar] [CrossRef]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of uniform polystyrene fibers: The effect of solvent conductivity. Polymer 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Feng, N.N.; Zhu, G.Y.; Niu, Y.W. Preparation and application of citral-monochlorotriazine-beta-cyclodextrin inclusion complex nanocapsule. J. Text. I. 2016, 107, 64–71. [Google Scholar] [CrossRef]





| Solutions | % CD a (w/v) | % citral b (w/w) | Viscosity (Pa·s) | Conductivity(μS/cm) | Average Fiber Diameter (AFD) (nm) | Fiber Morphology |
|---|---|---|---|---|---|---|
| Citral/HPβCD-IC-NF | 160 | 9.3 | 0.27 | 19.67 | 105 ± 35 | bead-free nanofibers |
| Citral/HPγCD-IC-NF | 160 | 8.4 | 0.37 | 8.26 | 1380 ± 380 | bead-free nanofibers |
| Citral/MβCD-IC-NF | 160 | 9.9 | 0.25 | 15.57 | 125 ± 35 | bead-free nanofibers |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aytac, Z.; Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral. Nanomaterials 2018, 8, 793. https://doi.org/10.3390/nano8100793
Aytac Z, Celebioglu A, Yildiz ZI, Uyar T. Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral. Nanomaterials. 2018; 8(10):793. https://doi.org/10.3390/nano8100793
Chicago/Turabian StyleAytac, Zeynep, Asli Celebioglu, Zehra Irem Yildiz, and Tamer Uyar. 2018. "Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral" Nanomaterials 8, no. 10: 793. https://doi.org/10.3390/nano8100793
APA StyleAytac, Z., Celebioglu, A., Yildiz, Z. I., & Uyar, T. (2018). Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral. Nanomaterials, 8(10), 793. https://doi.org/10.3390/nano8100793







