Homologous Gold Nanoparticles and Nanoclusters Composites with Enhanced Surface Raman Scattering and Metal Fluorescence for Cancer Imaging
Abstract
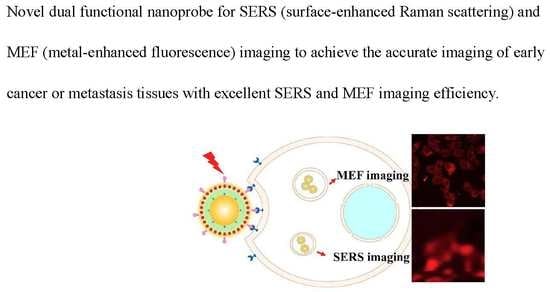
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the AuNP-MBA
2.3. Preparation of the AuNP@SiO2 and AuNPC
2.4. Conjugation of Targeted Molecule cRGD
2.5. Characterization
2.6. Biocompatibility and Cellular Uptake of Nanoparticles
2.7. Surface-Enhanced Raman Scattering (SERS) and Metal-Enhanced Fluorescence (MEF) Imaging
3. Results and Discussion
3.1. Characterization of the Dual Functional Nanoprobes
3.2. Biocompatibility and Cellular Uptake of Nanoparticles
3.3. Application of SERS and MEF Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA-Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kalluru, P.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Nano-graphene oxide-mediated in vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 2016, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, L.; Zeng, L.; Ren, W.; Xiao, X.; Zhang, J.; Zhang, L.; Li, A.; Lu, G.; Wu, A. Gd-based upconversion nanocarriers with yolk-shell structure for dual-modal imaging and enhanced chemotherapy to overcome multidrug resistance in breast cancer. Nanoscale 2016, 8, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, R.; Yang, C.; Zhang, X.; Zeng, Y.; Wang, S.; Guo, X.; Li, Y.; Cai, X.; Li, S.; et al. An ultrasensitive bioluminogenic probe of γ-glutamyltranspeptidase in vivo and in human serum for tumor diagnosis. Biosens. Bioelectron. 2017, 98, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaxton, C.S.; Rink, J.S.; Naha, P.C.; Cormode, D.P. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 116–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erami, R.; Ovejero, K.; Meghdadi, S.; Filice, M.; Amirnasr, M.; Rodríguez-Diéguez, A.; De La Orden, M.; Gómez-Ruiz, S. Applications of nanomaterials based on magnetite and mesoporous silica on the selective detection of zinc ion in live cell imaging. Nanomaterials 2018, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, W.; Liao, Z.; Yang, S.; Yang, S.; Li, X.; Zuo, F.; Luo, J. Role of Mn2+ doping in the preparation of core-shell structured Fe3O4@upconversion nanoparticles and their applications in T1/T2-weighted magnetic resonance imaging, upconversion luminescent imaging and near-infrared activated photodynamic therapy. Nanomaterials 2018, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Liu, Q.H.; Gao, D.L.; Luo, D.; Niu, Y.; Yang, J.; Li, Y. Graphene oxide as a multifunctional platform for Raman and fluorescence imaging of cells. Small 2015, 11, 3000–3005. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Chung, B.H. Preparation of pyrenyl-based multifunctional nanocomposites for biomedical applications. Nat. Protoc. 2016, 11, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.Y.; Lee, S.; Pyo, J.; Choo, J.; Lee, J.K. Bio-inspired development of a dual-mode nanoprobe for MRI and Raman imaging. Small 2015, 11, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ortiz, M.N.; Sentosun, K.; Bals, S.; Liz-Marzán, L.M. Templated growth of surface enhanced Raman scattering-active branched gold nanoparticles within radial mesoporous silica shells. ACS Nano 2015, 9, 10489–10497. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Khlebtsov, B.N.; Khanadeev, V.A.; Khlebtsov, N.G.; Ye, J. Rational design of ultrabright SERS probes with embedded reporters for bioimaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 30387–30397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath analysis based on surface-enhanced Raman scattering sensors distinguishes early and advanced gastric cancer patients from healthy persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chon, H.; Yoon, S.Y.; Lee, E.K.; Chang, S.I.; Lim, D.W.; Choo, J. Fabrication of SERS-fluorescence dual modal nanoprobes and application to multiplex cancer cell imaging. Nanoscale 2012, 4, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhu, T.; Liu, Z. Approaching the electromagnetic mechanism of surface-enhanced Raman scattering: From self-assembled arrays to individual gold nanoparticles. Chem. Soc. Rev. 2011, 40, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sheng, Z.; Li, P.; Wu, M.; Zhang, N.; Yu, X.F.; Wang, Y.; Hu, D.; Zheng, H.; Wang, G.P. Indocyanine green-loaded gold nanostars for sensitive SERS imaging and subcellular monitoring of photothermal therapy. Nanoscale 2017, 9, 11888–11901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, Y.K.; Fujita, T.; Zhang, Y.; Chen, M.W.; Wang, T.H. Large enhancement of quantum dot Fluorescence by highly scalable nanoporous gold. Adv. Mater. 2014, 26, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xiao, C.; Lau, W.-F.; Li, J.; Fu, J. Metal enhanced fluorescence improved protein and DNA detection by zigzag Ag nanorod arrays. Biosens. Bioelectron. 2016, 82, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.S.; Gao, N.Y.; Li, S.; Lang, M.J.; Xu, Q.H. Single-particle spectroscopic study on fluorescence enhancement by plasmon doupled gold nanorod dimers assembled on DNA origami. J. Phys. Chem. Lett. 2015, 6, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Hahn, L.D.; Yuen, W.W.; Vlamakis, H.; Kolter, R.; Mooney, D.J. Metal-enhanced fluorescence to quantify bacterial adhesion. Adv. Mater. 2011, 23, H101–H104. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xia, Y.; Huang, Y.; Li, J.; Ruan, H.; Chen, T.; Luo, L.; Shen, Z.; Wu, A. Improved SERS-active nanoparticles with various shapes for CTC detection without enrichment process with supersensitivity and high specificity. ACS Appl. Mater. Interfaces 2016, 8, 19928–19938. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, L.; Yang, S.; Ma, X.; Li, Y.; Dong, C.; Tian, Y.; Zhang, L.E.; Shen, Z.; Wu, A. Improved SERS nanoparticles for direct detection of circulating tumor cells in the blood. ACS Appl. Mater. Interfaces 2015, 7, 9965–9971. [Google Scholar] [CrossRef] [PubMed]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Xie, J.P.; Zheng, Y.G.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ouyang, J.; Sun, H.; Meng, F.; Cheng, R.; Zhang, J.; Cheng, L.; Lan, Q.; Deng, C.; Zhong, Z. Biodegradable micelles based on poly(ethylene glycol)-b-polylipopeptide copolymer: A robust and versatile nanoplatform for anticancer drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 27587–27595. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, B.; Cai, S.; Wang, P.; Peng, S.; Sheng, Y.; He, Y.; Gu, Y.; Chen, H. Dual targeting luminescent gold nanoclusters for tumor imaging and deep tissue therapy. Biomaterials 2016, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Hajebifard, A.; George, C.; Berini, P.; Zou, S. Ordered gold nanoparticle arrays on glass and their characterization. J. Colloid Interface Sci. 2013, 410, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Chakravadhanula, V.S.K.; Hrkac, V.; Jebril, S.; Agarwal, D.C.; Mohapatra, S.; Avasthi, D.K.; Kienle, L.; Adelung, R. Crystal growth behaviour in Au-ZnO nanocomposite under different annealing environments and photoswitchability. J. Appl. Phys. 2012, 112, 064308. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Meyers, S.; Shubeita, S.; Feldman, L.C.; Gustafsson, T.; Fabris, L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale 2014, 6, 8891–8899. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Adelung, R.; Kumar, G.; Elbahri, M.; Mohapatra, S.; Singhal, R.; Tripathi, A.; Avasthi, D.K. Formation of Self-organized Silver Nanocup-Type Structures and Their Plasmonic Absorption. Plasmonics 2013, 8, 811–815. [Google Scholar] [CrossRef]
- Yanamala, N.; Kagan, V.E.; Shvedova, A.A. Molecular modeling in structural nano-toxicology: Interactions of nano-particles with nano-machinery of cells. Adv. Drug Deliv. Rev. 2013, 65, 2070–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, K.; Wu, M.; Lakowicz, J.R.; Geddes, C.D. Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms. J. Am. Chem. Soc. 2007, 129, 1524–1525. [Google Scholar] [CrossRef] [PubMed]






| Nomenclature | CAu (mg/mL) | C4-MBA (μM) | SERS Intensity a |
|---|---|---|---|
| AuNP-MBA1 | 0.05 | 500 | 964 ± 2.5 |
| AuNP-MBA2 | 0.05 | 100 | 627 ± 5.2 |
| AuNP-MBA3 | 0.05 | 50 | 215 ± 6.7 |
| AuNP-MBA4 | 0.05 | 10 | 23 ± 4.3 |
| NO. | Size (nm) | The Thickness of Shell (nm) | MEF Intensity a |
|---|---|---|---|
| AuNPC1 | 45 ± 1.9 | 6.3 ± 0.8 | 930 ± 3.2 |
| AuNPC2 | 60 ± 2.3 | 13 ± 1.2 | 1545 ± 4.2 |
| AuNPC3 | 70 ± 2.9 | 17 ± 1.3 | 1480 ± 4.1 |
| AuNPC4 | 78 ± 4.0 | 20 ± 2.0 | 1037 ± 5.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Peng, Y.; Duan, X.; Yang, L.; Lan, J.; Wang, F. Homologous Gold Nanoparticles and Nanoclusters Composites with Enhanced Surface Raman Scattering and Metal Fluorescence for Cancer Imaging. Nanomaterials 2018, 8, 819. https://doi.org/10.3390/nano8100819
Wu X, Peng Y, Duan X, Yang L, Lan J, Wang F. Homologous Gold Nanoparticles and Nanoclusters Composites with Enhanced Surface Raman Scattering and Metal Fluorescence for Cancer Imaging. Nanomaterials. 2018; 8(10):819. https://doi.org/10.3390/nano8100819
Chicago/Turabian StyleWu, Xiaoxia, Yan Peng, Xiaomei Duan, Lingyan Yang, Jinze Lan, and Fu Wang. 2018. "Homologous Gold Nanoparticles and Nanoclusters Composites with Enhanced Surface Raman Scattering and Metal Fluorescence for Cancer Imaging" Nanomaterials 8, no. 10: 819. https://doi.org/10.3390/nano8100819
APA StyleWu, X., Peng, Y., Duan, X., Yang, L., Lan, J., & Wang, F. (2018). Homologous Gold Nanoparticles and Nanoclusters Composites with Enhanced Surface Raman Scattering and Metal Fluorescence for Cancer Imaging. Nanomaterials, 8(10), 819. https://doi.org/10.3390/nano8100819