Surface Coating for Flame Retardancy and Pyrolysis Behavior of Polyester Fabric Based on Calcium Alginate Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
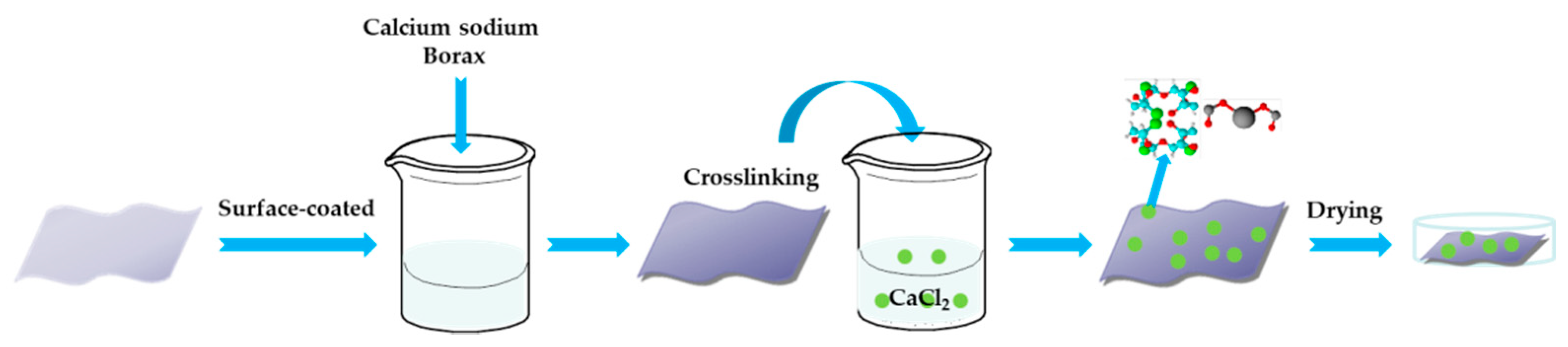
2.2. Preparation of Calcium Alginate/Nano-Calcium Borate Composites Coated Polyester Fabric
2.3. Measurements and Characterizations
2.3.1. Tensile Strength and Elongation
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Fourier Transform Infrared (FTIR)
2.3.4. Limiting Oxygen Index (LOI)
2.3.5. Vertical Burning Rate (UL-94)
2.3.6. Cone Calorimeter (CONE)
2.3.7. Thermogravimetric Analysis (TGA)
2.3.8. Pyrolysis-Gas Chromatograpgy-Mass Spectrometry (Py-GC-MS)
3. Results and Discussion
3.1. Tensile Strength and Elongation
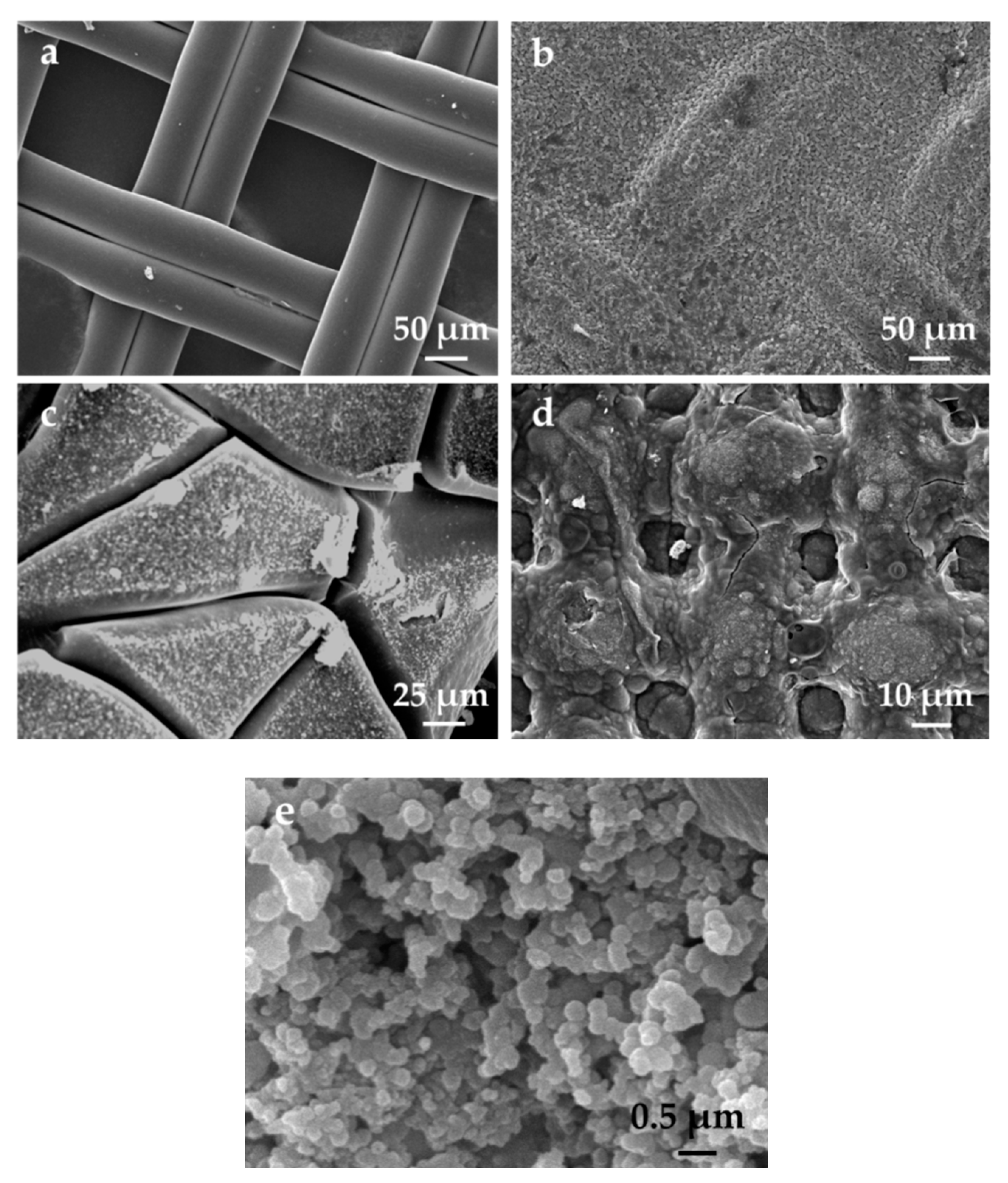
3.2. SEM
3.3. FTIR Analysis
3.4. Flame Retardancy and Combustion Behavior of the CAB-PL and PL
3.4.1. LOI
3.4.2. UL-94
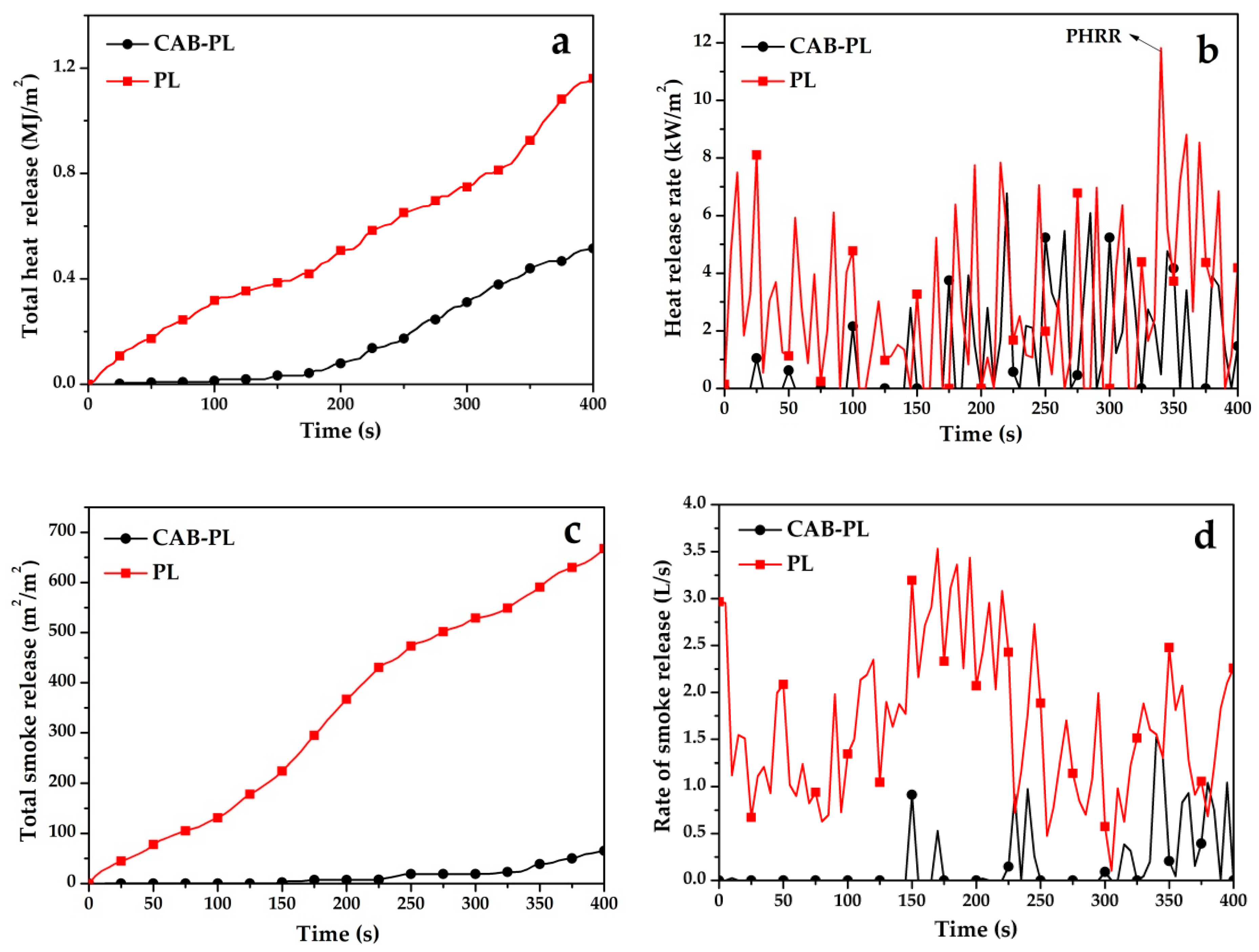
3.4.3. CONE
3.4.4. TGA and Differential Thermogravimetry (DTG)
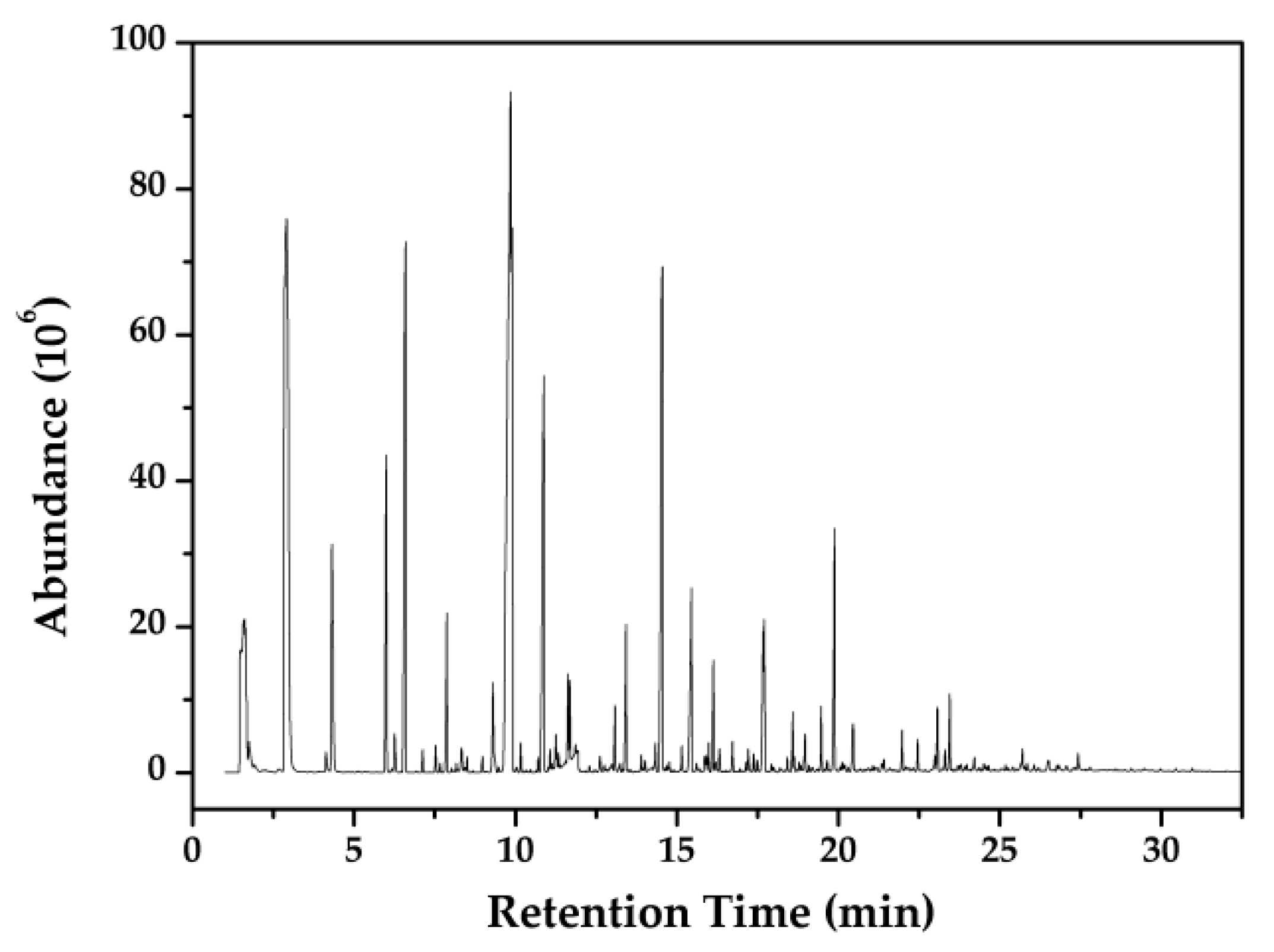
3.5. Py-GC-MS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radetić, M. Functionalization of textile materials with TiO2 nanoparticles. J. Photochem. Photobiol. C 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Xu, D.; He, Y.; Yu, Y.; Zhang, Q. Multiple parameter determination in textile material design: A bayesian inference approach based on simulation. Math. Comput. Simul. 2018, 151, 1–14. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Sun, G. Thermodynamics, kinetics, and multifunctional finishing of textile materials with colorants extracted from natural renewable sources. ACS Sustain. Chem. Eng. 2017, 5, 7451–7466. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Mohammad, F. High-energy radiation induced sustainable coloration and functional finishing of textile materials. Ind. Eng. Chem. Res. 2015, 54, 3727–3745. [Google Scholar] [CrossRef]
- Ma, J.-Y. Analysis on the fire risk existing in the storage of textile materials and textile goods. Procedia Eng. 2014, 71, 271–275. [Google Scholar] [CrossRef]
- Valverde, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Hydrolytic and enzymatic degradation studies of aliphatic 10-undecenoic acid-based polyesters. Polym. Degrad. Stab. 2018, 155, 84–94. [Google Scholar] [CrossRef]
- García, P.G.; Ramírez-Aguilar, R.; Torres, M.; Franco-Urquiza, E.A.; May-Crespo, J. Mechanical and thermal behavior dependence on graphite and oxidized graphite content in polyester composites. Polymer 2018, 153, 9–16. [Google Scholar] [CrossRef]
- Brännström, S.; Finnveden, M.; Johansson, M.; Martinelle, M.; Malmström, E. Itaconate based polyesters: Selectivity and performance of esterification catalysts. Eur. Polym. J. 2018, 103, 370–377. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Zhu, Y.J.; Yu, H.P.; Lu, B.Q. Biodegradable nanocomposite of glycerol citrate polyester and ultralong hydroxyapatite nanowires with improved mechanical properties and low acidity. J. Colloid Interface Sci. 2018, 530, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.N.; Lee, H.; Choi, D.; Kang, C.Y.; Im, S.S.; Kim, I.S. Fabrication of two polyester nanofiber types containing the biobased monomer isosorbide: poly (ethylene glycol 1,4-cyclohexane dimethylene isosorbide terephthalate) and poly (1,4-cyclohexane dimethylene isosorbide terephthalate). Nanomaterials 2018, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Kandola, B.K.; Ndiaye, M.; Price, D. Quantification of polymer degradation during melt dripping of thermoplastic polymers. Polym. Degrad. Stab. 2014, 106, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Ruch, D. Layer-by-layer deposition of a TiO2-filled intumescent coating and its effect on the flame retardancy of polyamide and polyester fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 1–10. [Google Scholar] [CrossRef]
- Gong, F.; Meng, C.; He, J.; Dong, X. Fabrication of highly conductive and multifunctional polyester fabrics by spray-coating with PEDOT:PSS solutions. Prog. Org. Coat. 2018, 121, 89–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Liu, X.; Huo, T.; Qin, Y. Preparation of durable flame retardant PAN fabrics based on amidoximation and phosphorylation. Appl. Surf. Sci. 2018, 428, 395–403. [Google Scholar] [CrossRef]
- Teixeira, M.; Sonnier, R.; Otazaghine, B.; Ferry, L.; Aubert, M.; Tirri, T.; Wilén, C.-E.; Rouif, S. Radiation-grafting of flame retardants on flax fabrics—A comparison between different flame retardant structures. Radiat. Phys. Chem. 2018, 145, 135–142. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Y.; Li, D.; He, S.; Zhang, F.; Zhang, G. A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohydr. Polym. 2017, 175, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Mateos, A.J.; Cain, A.A.; Grunlan, J.C. Large-scale continuous immersion system for layer-by-layer deposition of flame Rretardant and conductive nanocoatings on fabric. Ind. Eng. Chem. Res. 2014, 53, 6409–6416. [Google Scholar] [CrossRef]
- Chang, S.; Slopek, R.P.; Condon, B.; Grunlan, J.C. Surface coating for flame-retardant behavior of cotton fabric using a continuous layer-by-layer process. Ind. Eng. Chem. Res. 2014, 53, 3805–3812. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, W.; Zhou, S.; Wang, X.; Sheng, H.; Pan, Y.; Song, L.; Hu, Y. A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohydr. Polym. 2017, 166, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Guido, E.; Alongi, J.; Colleoni, C.; Di Blasio, A.; Carosio, F.; Verelst, M.; Malucelli, G.; Rosace, G. Thermal stability and flame retardancy of polyester fabrics sol–gel treated in the presence of boehmite nanoparticles. Polym. Degrad. Stab. 2013, 98, 1609–1616. [Google Scholar] [CrossRef]
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.L.; Hamers, T.; Kamstra, J.H.; Willmore, W.G.; Letcher, R.J. Organophosphate triesters and selected metabolites enhance binding of thyroxine to human transthyretin in vitro. Toxicol. Lett. 2018, 285, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Salmeia, K.A.; Gooneie, A.; Simonetti, P.; Nazir, R.; Kaiser, J.-P.; Rippl, A.; Hirsch, C.; Lehner, S.; Rupper, P.; Hufenus, R.; et al. Comprehensive study on flame retardant polyesters from phosphorus additives. Polym. Degrad. Stab. 2018, 155, 22–34. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Dai, Y.; Zhu, Y.; Wang, B.; Zhong, Y.; Zhang, L.; Sui, X.; Xu, H.; Mao, Z. Application of self-templated PHMA sub-microtubes in enhancing flame-retardance and anti-dripping of PET. Polym. Degrad. Stab. 2018, 154, 239–247. [Google Scholar] [CrossRef]
- Li, H.; Rosebrock, C.D.; Wu, Y.; Wriedt, T.; Mädler, L. Single droplet combustion of precursor/solvent solutions for nanoparticle production: Optical diagnostics on single isolated burning droplets with micro-explosions. Proc. Combust. Inst. 2018. [Google Scholar] [CrossRef]
- Wu, J.-N.; Chen, L.; Fu, T.; Zhao, H.-B.; Guo, D.-M.; Wang, X.-L.; Wang, Y.-Z. New application for aromatic Schiff base: High efficient flame-retardant and anti-dripping action for polyesters. Chem. Eng. J. 2018, 336, 622–632. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-protective and flame-retardant coatings—A state-of-the-art review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Kopacic, S.; Walzl, A.; Zankel, A.; Leitner, E.; Bauer, W. Alginate and chitosan as a functional barrier for paper-based packaging materials. Coatings 2018, 8, 235. [Google Scholar] [CrossRef]
- Mai, Z.; Xiong, Z.; Shu, X.; Liu, X.; Zhang, H.; Yin, X.; Zhou, Y.; Liu, M.; Zhang, M.; Xu, W.; et al. Multifunctionalization of cotton fabrics with polyvinylsilsesquioxane/ZnO composite coatings. Carbohydr. Polym. 2018, 199, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Bang, S.; Zhang, S.; Noh, I. Bioactive Molecules release and cellular responses of alginate-tricalcium phosphate particles hybrid gel. Nanomaterials 2017, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, X.; Chen, W.; Guo, W.; Chen, L.; Li, D. Hydroxyl radical pretreatment for low-viscosity sodium alginate production from brown seaweed. Algal Res. 2018, 34, 191–197. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Shi, R.; Tan, L.; Zong, L.; Ji, Q.; Li, X.; Zhang, K.; Cheng, L.; Xia, Y. Influence of Na+ and Ca2+ on flame retardancy, thermal degradation, and pyrolysis behavior of cellulose fibers. Carbohydr. Polym. 2017, 157, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Zhao, X.; Deng, Y.; Xue, Y.; Li, Q. Flame retardancy and thermal degradation mechanism of calcium alginate/CaCO3 composites prepared via in situ method. J. Therm. Anal. Calorim. 2017, 131, 2167–2177. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhao, X.; Zhang, L.; Li, Q. Highly efficient flame retardant hybrid composites based on calcium alginate/nano-calcium borate. Polymers 2018, 10, 625. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.H.; Guo, Y.N.; Gao, W.; Lu, X. FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China. J. Anal. Appl. Pyrolysis 2013, 100, 75–80. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Wang, X.; Zhang, H.; Zhang, Q.; Xiang, L. Short belt-like Ca2B2O5·H2O nanostructures: Hydrothermal formation, FT-IR, thermal decomposition, and optical properties. J. Cryst. Growth 2011, 332, 81–86. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Ni, A.; Ding, A.; Sun, Z.; Han, X. Effect of novel intumescent flame retardant on mechanical and flame retardant properties of continuous glass fibre reinforced polypropylene composites. Compos. Struct. 2018, 203, 894–902. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Liu, Y.; Cui, L.; Yan, C.; Zhu, P. Bio-based calcium alginate nonwoven fabrics: Flame retardant and thermal degradation properties. J. Anal. Appl. Pyrolysis 2016, 122, 13–23. [Google Scholar] [CrossRef]




| Samples | Tensile Strengh (N) | Elongation at Break (%) | LOI (%) | PHRR (Kw/m2) | TSR (MJ/m2) |
|---|---|---|---|---|---|
| CAB-PL | 592 | 26.9 | 34 | 6.8 | 86 |
| PL | 518 | 26.2 | 25 | 11.8 | 757 |
| Samples | CAB-PL | PL | ||
|---|---|---|---|---|
| Print cloth |  |  |  |  |
| After flame time (s) | 0 | 3.9 | ||
| After glow time (s) | 0 | 0 | ||
| Dripping | No | Yes | ||
| Rate | V-0 | N.R | ||
| S.N. | Molecular Structure | Name of Compound | Time | Area |
|---|---|---|---|---|
| 1 |  | carbon dioxide | 1.55 | 3.05 |
| 2 |  | 2,3-butanedione | 1.62 | 3.94 |
| 3 | 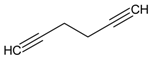 | 1,5-hexadiyne | 2.85 | 4.78 |
| 4 | 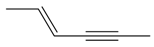 | 2-hexen-4-yne | 2.91 | 14.56 |
| 5 |  | methylbenzene | 4.32 | 3.17 |
| 6 | 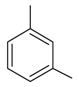 | 1,3-dimethylbenzen | 5.99 | 3.10 |
| 7 | 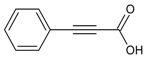 | phenylpropiolic acid | 6.26 | 0.39 |
| 8 |  | annulene | 6.59 | 7.90 |
| 9 | 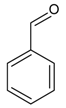 | benzaldehyde | 7.87 | 7.68 |
| 10 |  | indene | 9.31 | 0.72 |
| 11 | 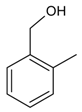 | 2-methylbenzyl alcohol | 9.84 | 21.90 |
| 12 | 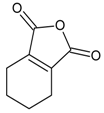 | 1,3-isobenzofurandione | 9.89 | 5.60 |
| 13 |  | 1-(4-methylphenyl)- acetophenone | 11.63 | 0.65 |
| 14 | 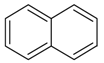 | naphthalene | 11.68 | 0.57 |
| 15 |  | 1-(4-ethylphenyl)- acetophenone | 13.08 | 0.54 |
| 16 | 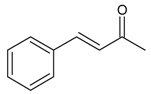 | 4-benzalacetone | 13.42 | 1.32 |
| 17 | 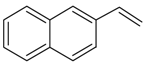 | 2-ethenyl-naphthalene | 14.55 | 8.87 |
| 18 | 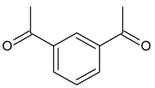 | 1,3-diacetylbenzene | 15.45 | 3.32 |
| 19 | 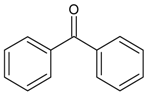 | benzophenone | 17.67 | 1.53 |
| 20 |  | 1,1′-diphenylethylene | 17.71 | 1.43 |
| 21 | 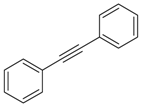 | diphenylacetylene | 19.46 | 0.49 |
| 22 |  | 4-phenylacetophenone | 19.89 | 2.85 |
| 23 | 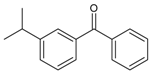 | (3-isopropylphenyl) (phenyl) methanone | 21.98 | 0.33 |
| 24 |  | (3-isopropylphenyl) (phenyl) methanone | 22.45 | 0.27 |
| 25 | 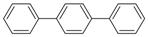 | p-terphenyl | 23.45 | 0.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, J.; Zhao, X.; Li, Z.; Li, Q. Surface Coating for Flame Retardancy and Pyrolysis Behavior of Polyester Fabric Based on Calcium Alginate Nanocomposites. Nanomaterials 2018, 8, 875. https://doi.org/10.3390/nano8110875
Liu Z, Li J, Zhao X, Li Z, Li Q. Surface Coating for Flame Retardancy and Pyrolysis Behavior of Polyester Fabric Based on Calcium Alginate Nanocomposites. Nanomaterials. 2018; 8(11):875. https://doi.org/10.3390/nano8110875
Chicago/Turabian StyleLiu, Zhenhui, Jiao Li, Xihui Zhao, Zichao Li, and Qun Li. 2018. "Surface Coating for Flame Retardancy and Pyrolysis Behavior of Polyester Fabric Based on Calcium Alginate Nanocomposites" Nanomaterials 8, no. 11: 875. https://doi.org/10.3390/nano8110875






