Rhodium Oxide Surface-Loaded Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Direct Current (DC) Resistance Measurements
2.3. DRIFT Spectroscopy
2.4. XRD Measurements
2.5. X-ray Absorption Spectroscopy (XAS) Measurements
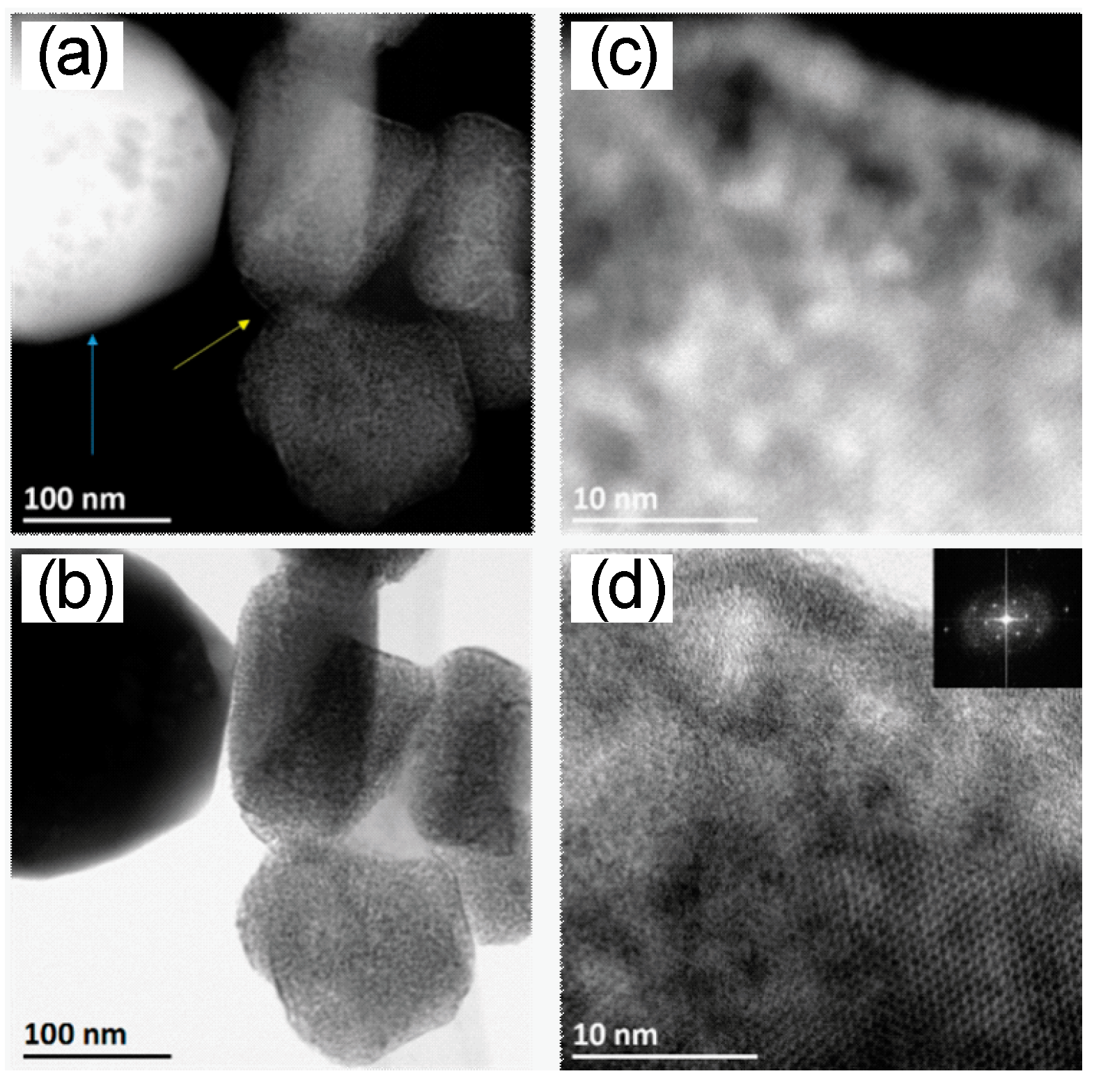
2.6. Scanning Transmission Electron Microscopy
3. Results
3.1. Material Characterization
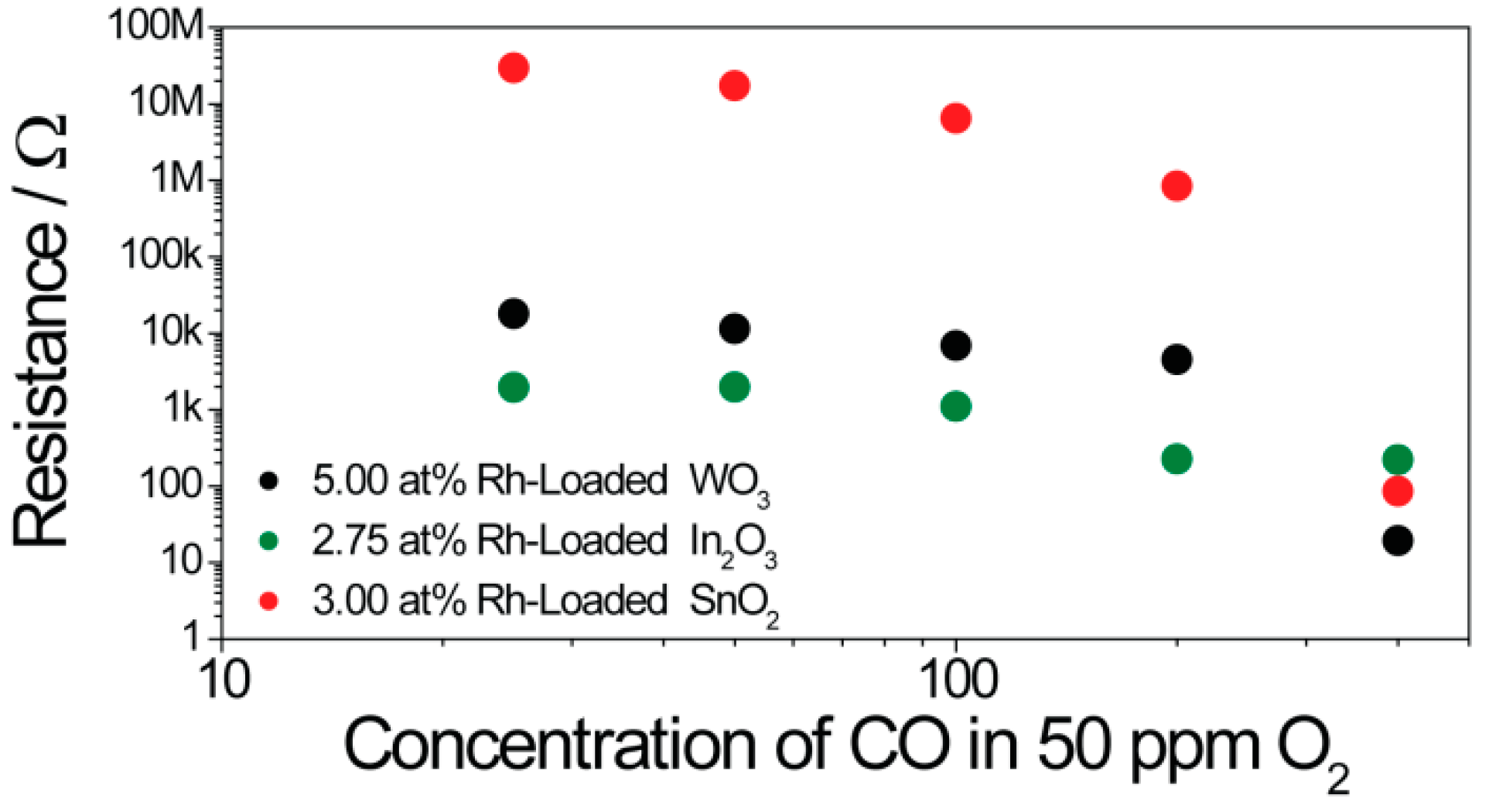
3.2. Resistance Measurements
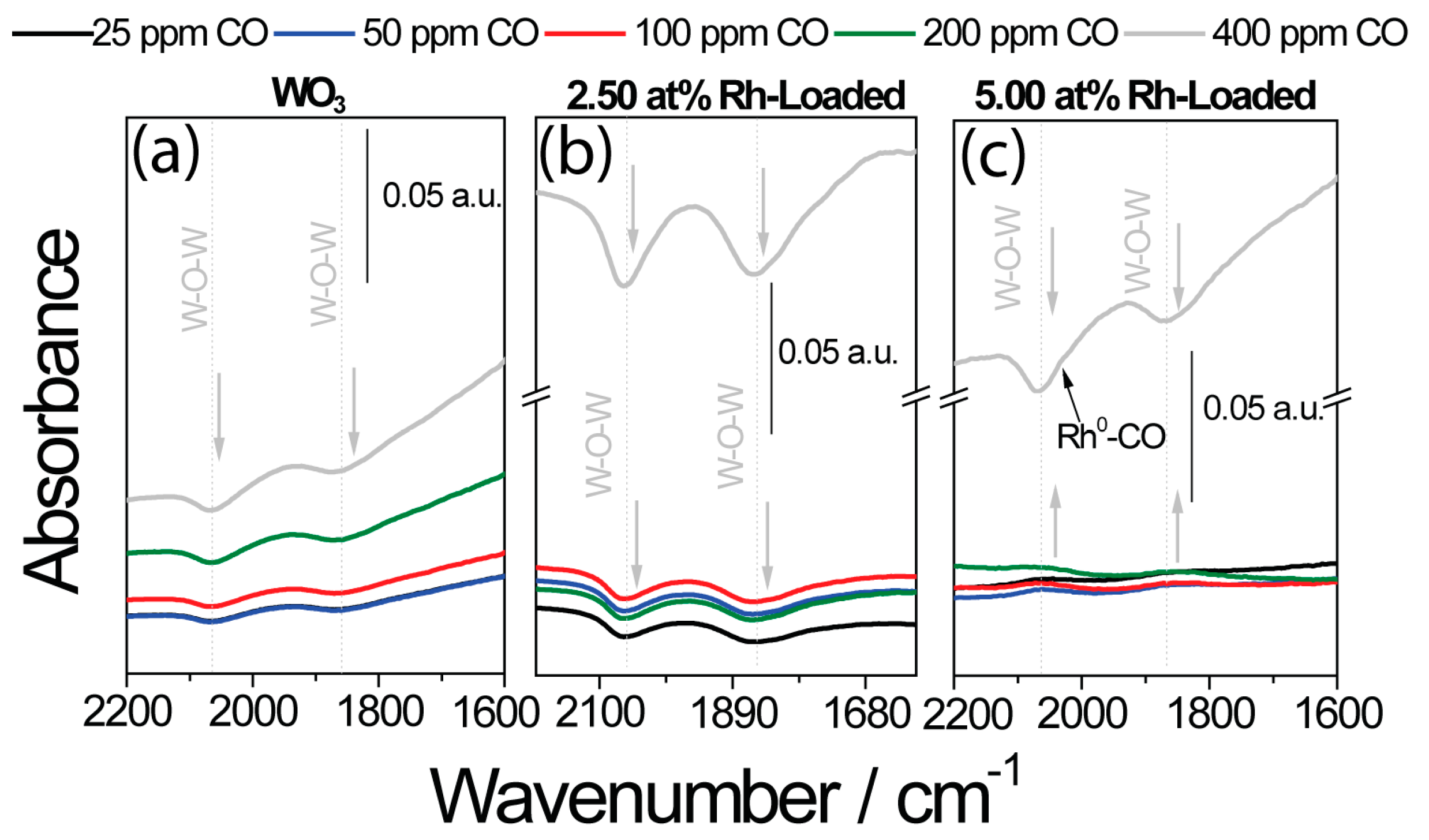
3.3. DRIFT Measurements
3.4. X-ray Absorption Spectroscopy (XAS)
4. Discussion and Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuator B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Chemical and Biochemical Sensors. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley VCH GmbH & Co KGaA. Posted September 29, 2016. Available online: https://doi.org/10.1002/14356007.b06_121.pub2 (accessed on 17 August 2018).
- Hoa, N.D.; Duy, N.V.; El-Safty, S.A.; Hieu, N.V. Meso-/nanoporous semiconducting metal oxides for gas sensor applications. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuator B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Hoa, N.D.; El-Safty, S.A. Highly sensitive and selective volatile organic compound gas sensors based on mesoporous nanocomposite monoliths. Anal. Methods 2011, 3, 1948–1956. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, N. Gas-Detecting Device. US3695848, 3 October 1972. [Google Scholar]
- Yamazoe, N.; Kurokawa, Y.; Seiyama, T. Effects of Additives on Semiconductor Gas Sensors. Sens. Actuators B 1983, 4, 283–289. [Google Scholar] [CrossRef]
- Morrison, S.R. Selectivity in semiconductor gas sensors. Sens. Actuators 1987, 12, 425–440. [Google Scholar] [CrossRef]
- Yamazoe, N. New Approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Matsushima, S.; Teraoka, Y.; Miura, N.; Yamazoe, N. Electronic interaction between metal additives and tin dioxide in tin dioxide-based gas sensors. JPN J. Appl. Phys. 1988, 27, 1798–1802. [Google Scholar] [CrossRef]
- Staerz, A.; Kim, T.-H.; Lee, J.-H.; Weimar, U.; Barsan, N. Nanolevel control of gas sensing characteristics via p–n heterojunction between Rh2O3 clusters and WO3 crystallites. J. Phys. Chem. C 2017, 121, 24701–24706. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Wiegelb, G.; Heitbaum, J. Semiconductor gas sensor for detecting NO and CO traces in ambient air of road traffic. Sens. Actuators B 1994, 17, 93–99. [Google Scholar] [CrossRef]
- Nakagawa, H.; Okazaki, S.; Asakura, S.; Fukuda, K.; Akimoto, H.; Takahashi, H.; Shigemori, S. An automated car ventilation system. Sens. Actuators B 2000, 65, 133–137. [Google Scholar] [CrossRef]
- Guidelines for indoor air quality. WHO Regional Office for Europe. 2010. Available online: http://www.euro.who.int/__data/assets/pdf_file/0009/128169/e94535.pdf (accessed on 21 July 2017).
- Residential Indoor Air Quality Guidelines. Government of Canada. 2018. Available online: http://healthycanadians.gc.ca/healthy-living-vie-saine/environment-environnement/air/guidelines-lignes-directrices-eng.php#a1 (accessed on 16 October 2018).
- Choi, K.-I.; Hwang, S.-J.; Dai, Z.; Kang, Y.C.; Lee, J.-H. Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics. RSC Adv. 2014, 4, 53130–53136. [Google Scholar] [CrossRef]
- Wicker, S.; Guiltat, M.; Weimar, U.; Hémeryck, A.; Barsan, N. Ambient humidity influence on CO detection with SnO2 gas sensing materials. A combined DRIFTS/DFT Investigation. J. Phys. Chem. C 2017, 121, 25064–25073. [Google Scholar] [CrossRef]
- Harris, D.C. Measurements. In Quantitative Chemical Analysis, 7th ed.; China Lake: Kern County, CA, USA, 2007; pp. 13–15. [Google Scholar]
- Degler, D.S.; Müller, A.; Doronkin, D.E.; Wang, D.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. Platinum loaded Tin dioxide: A model system for unravelling the interplay between heterogeneous catalysis and gas sensing. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Bârsan, N.; Hübner, M.; Weimar, U. Conduction mechanisms in SnO2 based polycrystalline thick film gas sensors exposed to CO and H2 in different oxygen backgrounds. Sens. Actuators B 2011, 157, 510–517. [Google Scholar] [CrossRef]
- Koziej, D.; Hübner, M.; Barsan, N.; Weimar, U.; Sikora, M.; Grunwaldt, J.-D. Operando X-ray absorption spectroscopy studies on Pd-SnO2 based sensors. Phys. Chem. Chem. Phys. 2009, 11, 8620–8625. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B. Quantitative EXAFS Analysis; van Bokhoven, J.A., Lamberti, C., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 281–302. [Google Scholar]
- Baur, W.H.; Khan, A. Rutile-type compounds. IV. SiO2, GeO2 and a comparison with other rutile-type structures. Acta Crystallogr. B 1971, 27, 2133–2139. [Google Scholar] [CrossRef]
- Loopstra, B.O.; Boldrini, P. Neutron diffraction inverstigation of WO3. Acta Crystallogr. B 1966. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Understanding the Potential of WO3 Based Sensors for Breath Analysis. Sensors 2016, 16, 1815. [Google Scholar] [CrossRef] [PubMed]
- Salje, E. The Orthorhombic Phase of WO3. Acta Crystallogr. B 1977, 33, 574–577. [Google Scholar] [CrossRef]
- Marezio, M. Refinement of the crystal structure of In2O3. Acta Crystallogr. B 1966, 20, 723–728. [Google Scholar] [CrossRef]
- Prewitt, C.T.; Shannon, R.D.; Rogers, D.B.; Sleight, A.W. The C rare earth oxide-corundum transition and crystal chemistry of oxides having the corundum structure. Inorg. Chem. 1969, 8, 1985–1993. [Google Scholar] [CrossRef]
- Birkholz, M. A Thin Film Analysis by X-ray Scattering; Wiley-VCH Verlag: Weinheim, Germany, 2006. [Google Scholar]
- Kuzmin, A.; Chaboy, J. EXAFS and XANES analysis of oxides at the nanoscale. IUCrJ 2014, 1, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anton, M.; Buedy, B. The influence of rhodium on SnO2-CO gas sensor. Sens. Actuators B 1994, 19, 500–501. [Google Scholar] [CrossRef]
- Brinkmann, H. An Investigation of Rh2O3-Loaded n-Type Semiconducting Metal Oxide Sensors. Teacher’s Thesis, University of Tuebingen, Tuebingen, Germany, 2017. [Google Scholar]
- Herrmann, S. Eine untersuchung von Rh2O3 geladenen In2O3 Gassensoren. Teacher’s Thesis, University of Tuebingen, Tuebingen, Germany, 2018. [Google Scholar]
- Weiss, B.M.; Artioli, N.; Iglesia, E. Catalytic NO oxidation pathways and redox cycles on dispersed oxides of rhodium and cobalt. ChemCatChem 2012, 4, 1397–1404. [Google Scholar] [CrossRef]
- Watson, P.R.; Somorjai, G.A. Interaction of carbon monoxide, carbon dioxide, and deuterium with rhodium oxide: its reduction and catalytic stability. J. Phys. Chem. 1982, 20, 3993–3996. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Mijalković, S. Optical properties and photoelectric effects. In Semiconductor Physical Electronics; Li, S.S., Ed.; Springer: New York, NY, USA, 1997; Chapter 9; pp. 248–256. [Google Scholar]
- Harrick, N.J. Optical spectrum of the semiconductor surface states from frustrated total internal reflections. Phys. Rev. 1962, 125, 1165–1170. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Haensch, A.; Barsan, N.; Weimar, U. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B 2010, 151, 103–106. [Google Scholar] [CrossRef]
- Can, I.; Weimar, U.; Barsan, N. Operando investigations of differently prepared In2O3-Gas sensors. Proceedings 2017, 1, 432. [Google Scholar] [CrossRef]
- Boehme, I.; Herrmann, S.; Staerz, A.; Brinkmann, H.; Weimar, U. Understanding the sensing mechanism of Rh2O3 loaded In2O3. Proceedings 2018, in press. [Google Scholar]
- Hecker, W.C.; Rasband, P.B. Catalyst characterization using quantitative FTIR: CO on supported Rh. J. Catal. 1993, 139, 551–560. [Google Scholar]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the active oxygen species in SnO2 based gas sensing materials: An operando IR spectrsocopy study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Amalric Popescu, D.; Herrmann, J.-M.; Ensuque, A.; Bozon-Verduraz, F. Nanosized tin dioxide: Spectroscopic (UV–VIS, NIR, EPR) and electrical conductivity studies. Phys. Chem. Chem. Phys. 2001, 3, 2522–2530. [Google Scholar] [CrossRef]
- Wicker, S. Influence of Humidity on the Gas Sensing Characteristics of SnO2: DRIFTS Investigation of Different Base Materials and Dopants. Ph.D. Thesis, University of Tuebingen, Tuebingen, Germany, 2016. [Google Scholar]
- De Groot, F. High-resolution X-ray emission and X-ray absorption spectroscopy. Chem. Rev. 2001, 101, 1779–1808. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.K.; Lima, F.A.; Pollock, C.J.; Rees, J.A.; DeBeer, S. A practical guide to high-resolution X-ray spectroscopic measurements and their applications in bioinorganic chemistry. Isr. J. Chem. 2016, 56, 803–815. [Google Scholar] [CrossRef]
- Koningsberger, D.C.; Prins, R. X-ray Absorption: Principles, Applications, Techniques of EXAFS, SEXAFS and XANES; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Grunwaldt, J.D.; Basini, L.; Clausen, B.S. In situ EXAFS study of Rh/Al2O3 catalysts for catalytic partial oxidation of methane. J. Catal. 2001, 200, 321–329. [Google Scholar] [CrossRef]
- Basini, L.; Marchionna, M.; Aragno, A. Drift and mass spectroscopic studies on the reactivity of rhodium clusters at the surface of polycrystalline oxides. J. Phys. Chem. 1992, 96, 9431–9441. [Google Scholar] [CrossRef]









| Sample | Atom | N | R | σ2 | S02 | δE0 | ρ |
|---|---|---|---|---|---|---|---|
| (Å) | (10−3 Å2) | (eV) | (%) | ||||
| 5.0 at.% Rh WO3 | O | 6.0 f | 2.02 ± 0.01 | 4.01 ± 1.75 | 0.72 ± 0.09 | −2.10 ± 1.14 | 1.7 |
| Rh | 2.0 f | 3.09 ± 0.02 | 5.25 ± 2.52 | ||||
| 3.0 at.% Rh SnO2 | O | 5.5 f | 2.04 ± 0.01 | 3.22 ± 1.38 | 0.84 ± 0.08 | −0.65 ± 0.94 | 1.1 |
| Rh | 2.0 f | 3.14 ± 0.03 | 4.39 ± 3.75 | ||||
| Rh | 2.5 f | 3.34 ± 0.03 | |||||
| 2.75 at.% Rh In2O3 | O | 5.5 f | 2.04 ± 0.01 | 3.45 ± 1.18 | 0.72 ± 0.06 | −1.14 ± 0.86 | 0.9 |
| Rh | 1.5 f | 3.15 ± 0.03 | 5.95 ± 4.41 | ||||
| Rh | 1.5 f | 3.38 ± 0.04 |
| Material | Band-bending (meV) |
|---|---|
| 2.50 at.% Rh-Loaded WO3 | ca. 198 |
| 5.00 at.% Rh-Loaded WO3 | ca. 200 |
| 0.50 at.% Rh-Loaded In2O3 | ca. 105 |
| 2.75 at.% Rh-Loaded In2O3 | ca. 98 |
| 0.50 at.% Rh-Loaded SnO2 | ca. 119 |
| 3.00 at.% Rh-Loaded SnO2 | ca. 222 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staerz, A.; Boehme, I.; Degler, D.; Bahri, M.; Doronkin, D.E.; Zimina, A.; Brinkmann, H.; Herrmann, S.; Junker, B.; Ersen, O.; et al. Rhodium Oxide Surface-Loaded Gas Sensors. Nanomaterials 2018, 8, 892. https://doi.org/10.3390/nano8110892
Staerz A, Boehme I, Degler D, Bahri M, Doronkin DE, Zimina A, Brinkmann H, Herrmann S, Junker B, Ersen O, et al. Rhodium Oxide Surface-Loaded Gas Sensors. Nanomaterials. 2018; 8(11):892. https://doi.org/10.3390/nano8110892
Chicago/Turabian StyleStaerz, Anna, Inci Boehme, David Degler, Mounib Bahri, Dmitry E. Doronkin, Anna Zimina, Helena Brinkmann, Sina Herrmann, Benjamin Junker, Ovidiu Ersen, and et al. 2018. "Rhodium Oxide Surface-Loaded Gas Sensors" Nanomaterials 8, no. 11: 892. https://doi.org/10.3390/nano8110892
APA StyleStaerz, A., Boehme, I., Degler, D., Bahri, M., Doronkin, D. E., Zimina, A., Brinkmann, H., Herrmann, S., Junker, B., Ersen, O., Grunwaldt, J.-D., Weimar, U., & Barsan, N. (2018). Rhodium Oxide Surface-Loaded Gas Sensors. Nanomaterials, 8(11), 892. https://doi.org/10.3390/nano8110892








