Controlling the Amorphous and Crystalline State of Multinary Alloy Nanoparticles in An Ionic Liquid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sputter Deposition
2.3. Materials Characterization
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Stepanov, A.L. Nonlinear optical properties of metal nanoparticles in silicate glass. Glas Nanocomposites 2016, 165–179. [Google Scholar]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2014, 140, 386–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, W. Inorganic nanomaterials for printed electronics: A review. Nanoscale 2017, 9, 7342–7372. [Google Scholar] [CrossRef] [PubMed]
- Scholten, J.D.; Leal, C.; Dupont, J. Transition metal nanoparticle catalysis in ionic liquids. ACS Catal. 2012, 2, 184–200. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Goesmann, H.; Feldmann, C. Nanoparticulate functional materials. Angewandte 2010, 49, 1362–1395. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, L.L.; Riche, C.T.; Malmstadt, N.; Brutchey, R.L. Effect of ionic liquid impurities on the synthesis of silver nanoparticles. Langmuir 2012, 28, 15987–15993. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, X.; Hedrick, J.L.; Xie, Z.; Wang, S.; Lin, Q.-Y.; Hersam, M.C.; Dravid, V.P.; Mirkin, C.A. Polyelemental nanoparticle libraries. Science 2016, 352, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, T.; Kameyama, T.; Kuwabata, S. Top-down synthesis methods for nanoscale catalysts. Nanocatalysis Ion. Liq. 2016, 171–205. [Google Scholar]
- He, Z.; Alexandridis, P. Nanoparticles in ionic liquids: Interactions and organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef] [PubMed]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering deposition of nanoparticles onto liquid substrates: Recent advances and future trends. Coord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Torimoto, T.; Okazaki, K.; Kiyama, T.; Hirahara, K. Sputter deposition onto ionic liquids: Simple and clean synthesis of highly dispersed ultrafine metal nanoparticles. Appl. Phys. Lett. 2006, 84, 243117. [Google Scholar] [CrossRef]
- Meyer, H.; Meischein, M.; Ludwig, A. Rapid assessment of sputtered nanoparticle ionic liquid combinations. ACS Comb. Sci. 2018, 20, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Vanecht, E.; Binnemans, K.; Seo, J.W.; Stappers, L.; Fransaer, J. Growth of sputter-deposited gold nanoparticles in ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 13565–13571. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Okazaki, K.; Kiyama, T.; Kuwabata, S.; Torimoto, T. A facile synthesis of AuAg alloy nanoparticles using a chemical reaction induced by sputter deposition of metal onto ionic liquids. Electrochemistry 2009, 77, 636–638. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, T.; Tomita, Y.; Hirano, M.; Okazaki, K.I.; Kuwabata, S.; Torimoto, T. Compositional control of AuPt nanoparticles synthesized in ionic liquids by the sputter deposition technique. Cryst. Eng. Comm. 2012, 14, 4922–4926. [Google Scholar] [CrossRef]
- Khatri, O.P.; Adachi, K.; Murase, K.; Okazaki, K.; Torimoto, T.; Tanaka, N.; Kuwabata, S.; Sugimura, H. Self-assembly of ionic liquid (BMI-PF6)-stabilized gold nanoparticles on a silicon surface: Chemical and structural aspects. Langmuir. 2008, 24, 7785–7792. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Kiyama, T.; Suzuki, T.; Kuwabata, S.; Torimoto, T. Thermally induced self-assembly of gold nanoparticles sputter-deposited in ionic liquids on highly ordered pyrolytic graphite surfaces. Chem. Lett. 2009, 38, 330–331. [Google Scholar] [CrossRef]
- Technol, J.E.S.; Cha, I.Y.; Yoo, S.J.; Jang, J.H. Recent progress in nanoparticle synthesis via liquid medium sputtering and its applications. J. Phys. Chem. 2016, 7, 13–26. [Google Scholar]
- König, D.; Richter, K.; Siegel, A.; Mudring, A.; Ludwig, A. High-throughput fabrication of Au–Cu nanoparticle libraries by combinatorial sputtering in ionic liquids. Adv. Funct. Mater. 2014, 24, 2049–2056. [Google Scholar] [CrossRef]
- Purposes, C. Bimetallic nanoparticles in alternative solvents for catalytic purposes. Catalysts 2017, 7, 207. [Google Scholar]
- Wender, H.; De Oliveira, L.F.; Migowski, P.; Feil, A.F.; Lissner, E.; Prechtl, M.H.G.; Teixeira, S.R.; Dupont, J. Ionic liquid surface composition controls the size of gold nanoparticles prepared by sputtering deposition. J. Phys. Chem. 2010, 114, 11764–11768. [Google Scholar] [CrossRef]
- Bohlmark, J.; Alami, J.; Christou, C.; Ehiasarian, A.P.; Helmersson, U. Ionization of sputtered metals in high power pulsed magnetron sputtering. J. Vac. Sci. Technol. 2005, 23, 18–22. [Google Scholar] [CrossRef]
- Löffler, T.; Meyer, H.; Savan, A.; Wilde, P.; Garzón-Manjón, A.; Chen, Y.T.; Ventosa, E.; Scheu, C.; Ludwig, A.; Schuhmann, W. Discovery of a multinary nobel metal free oxygen reduction catalyst. Advanced Energy Mater. 2018. [Google Scholar] [CrossRef]
- Zhang, T.; Song, Z.; Sun, M.; Liu, B.; Feng, S.; Chen, B. Investigation of electron beam induced phase change in Si2Sb2Te5 material. Appl. Phys. A. 2008, 90, 451–455. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy: A Textbook for Materials Science, 2nd Edition ed; Springer Science+Business Media: New York, NY, USA, 2009. [Google Scholar]
- Lv, Z.Y.; Liu, X.J.; Jia, B.; Wang, H.; Wu, Y.; Lu, Z.P. Development of a novel high- entropy alloy with eminent efficiency of degrading azo dye solutions. Sci. Rep. 2016, 6, 34213. [Google Scholar] [CrossRef] [PubMed]
- Mandegarzad, S.; Raoof, J.B.; Hosseini, S.R.; Ojani, R. Cu-Pt bimetallic nanoparticles supported metal organic framework-derived nanoporous carbon as a catalyst for hydrogen evolution reaction. Electrochim. Acta 2016, 190, 729–736. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Huang, Q.; Li, X.; Zou, Z.; Yang, H. Methanol oxidation on carbon-supported Pt-Ru-Ni ternary nanoparticle electrocatalysts. J. Power Sources 2008, 175, 159–165. [Google Scholar] [CrossRef]
- Alami, J.; Eklund, P.; Andersson, J.M.; Lattemann, M.; Wallin, E.; Bohlmark, J.; Persson, P.; Helmersson, U. Phase tailoring of Ta thin films by highly ionized pulsed magnetron sputtering. Thin Solid Films 2007, 515, 3434–3438. [Google Scholar] [CrossRef]
- Wallin, E.; Selinder, T.I.; Elfwing, M.; Helmersson, U. Synthesis of α-Al2O3 thin films using reactive high-power impulse magnetron sputtering. EPL 2008, 82, 36002 1-5. [Google Scholar] [CrossRef]
- Selinder, T.I.; Coronel, E.; Wallin, E.; Helmersson, U. α-Alumina coatings on WC/Co substrates by physical vapor deposition. Int. J. Refract. Met. Hard Mater. 2009, 27, 507–512. [Google Scholar] [CrossRef]
- Alami, J.; Sarakinos, K.; Uslu, F.; Klever, C.; Dukwen, J.; Wuttig, M. On the phase formation of titanium oxide films grown by reactive high power pulsed magnetron sputtering. J. Phys. D Appl. Phys. 2009, 42, 115204. [Google Scholar] [CrossRef]
- Aiempanakit, M.; Helmersson, U.; Aijaz, A.; Larsson, P.; Magnusson, R.; Jensen, J.; Kubart, T. Effect of peak power in reactive high power impulse magnetron sputtering of titanium dioxide. Surf. Coat. Technol. 2011, 205, 4828–4831. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Kauffmann, A.; Stüber, M.; Leiste, H.; Ulrich, S.; Schlabach, S.; Vinga, D.; Seils, S.; Gorr, B.; Chen, H.; Seifert, H.; et al. Combinatorial exploration of the high entropy alloy system Co-Cr-Fe-Mn-Ni. Surf. Coat. Technol. 2017, 325, 174–180. [Google Scholar] [CrossRef]




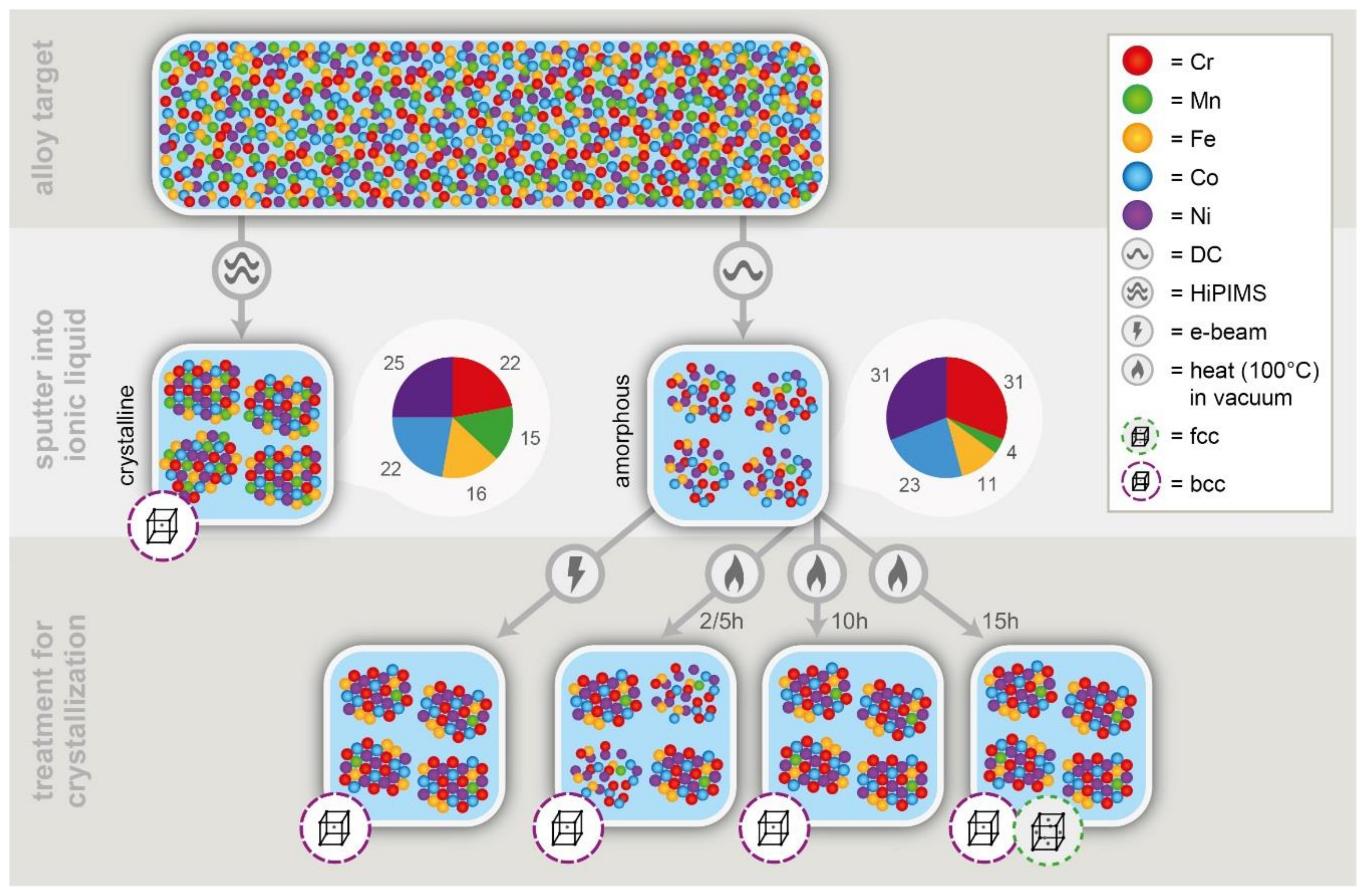
| Element | Dc Sputtered | HiPIMS Sputtered | |||
|---|---|---|---|---|---|
| As-Deposited in at% | 15 h of Annealed in at% | Overall in at% | As-Deposited in at% | Overall in at% | |
| Cr | 33–46 | 17-27 | 31* | 15–20 | 22* |
| Mn | 1–3 | 5-8 | 4* | 10–19 | 15* |
| Fe | 11–15 | 15-17 | 11* | 19–26 | 16* |
| Co | 23–26 | 31-36 | 23* | 26–31 | 22* |
| Ni | 18–30 | 19-24 | 31* | 13–21 | 25* |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzón-Manjón, A.; Meyer, H.; Grochla, D.; Löffler, T.; Schuhmann, W.; Ludwig, A.; Scheu, C. Controlling the Amorphous and Crystalline State of Multinary Alloy Nanoparticles in An Ionic Liquid. Nanomaterials 2018, 8, 903. https://doi.org/10.3390/nano8110903
Garzón-Manjón A, Meyer H, Grochla D, Löffler T, Schuhmann W, Ludwig A, Scheu C. Controlling the Amorphous and Crystalline State of Multinary Alloy Nanoparticles in An Ionic Liquid. Nanomaterials. 2018; 8(11):903. https://doi.org/10.3390/nano8110903
Chicago/Turabian StyleGarzón-Manjón, Alba, Hajo Meyer, Dario Grochla, Tobias Löffler, Wolfgang Schuhmann, Alfred Ludwig, and Christina Scheu. 2018. "Controlling the Amorphous and Crystalline State of Multinary Alloy Nanoparticles in An Ionic Liquid" Nanomaterials 8, no. 11: 903. https://doi.org/10.3390/nano8110903
APA StyleGarzón-Manjón, A., Meyer, H., Grochla, D., Löffler, T., Schuhmann, W., Ludwig, A., & Scheu, C. (2018). Controlling the Amorphous and Crystalline State of Multinary Alloy Nanoparticles in An Ionic Liquid. Nanomaterials, 8(11), 903. https://doi.org/10.3390/nano8110903






