Effect of Conductive Material Morphology on Spherical Lithium Iron Phosphate
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of LiFePO4/C Battery
2.2. Material Characterization
2.3. Electrochemical Analysis
3. Results and Discussion
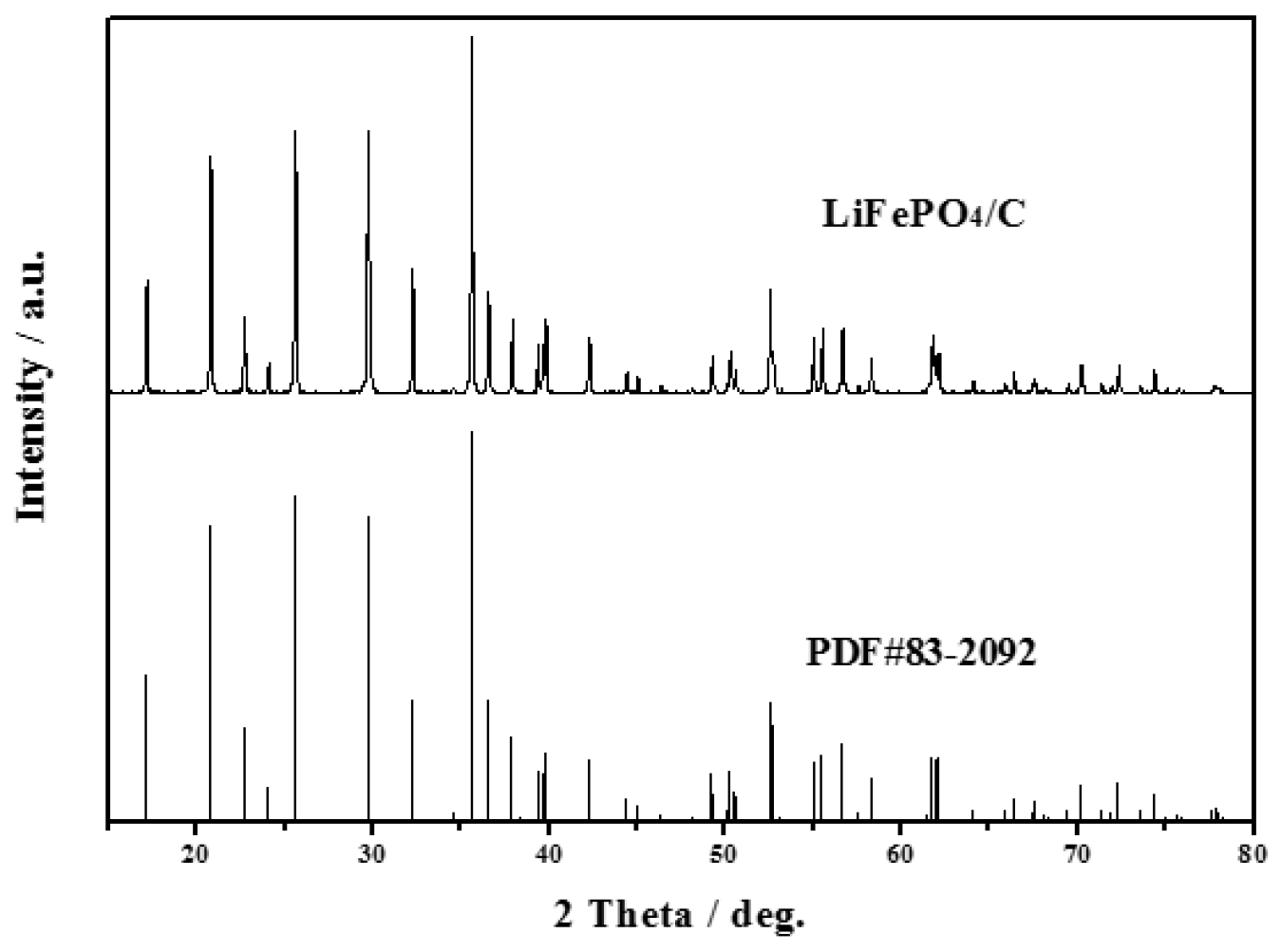
3.1. Characterization of LiFePO4/C Composite
3.2. Processability of Electrodes
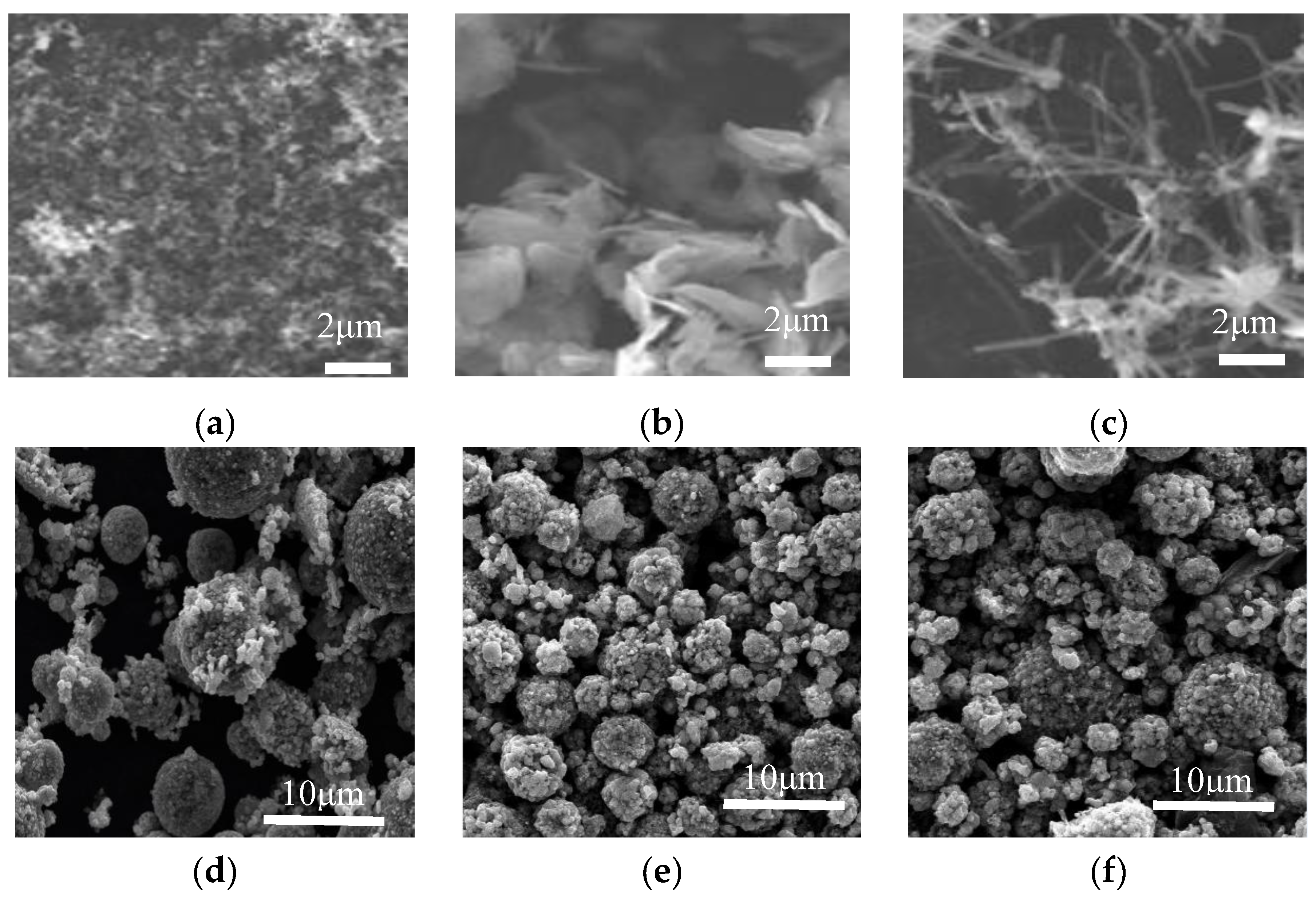
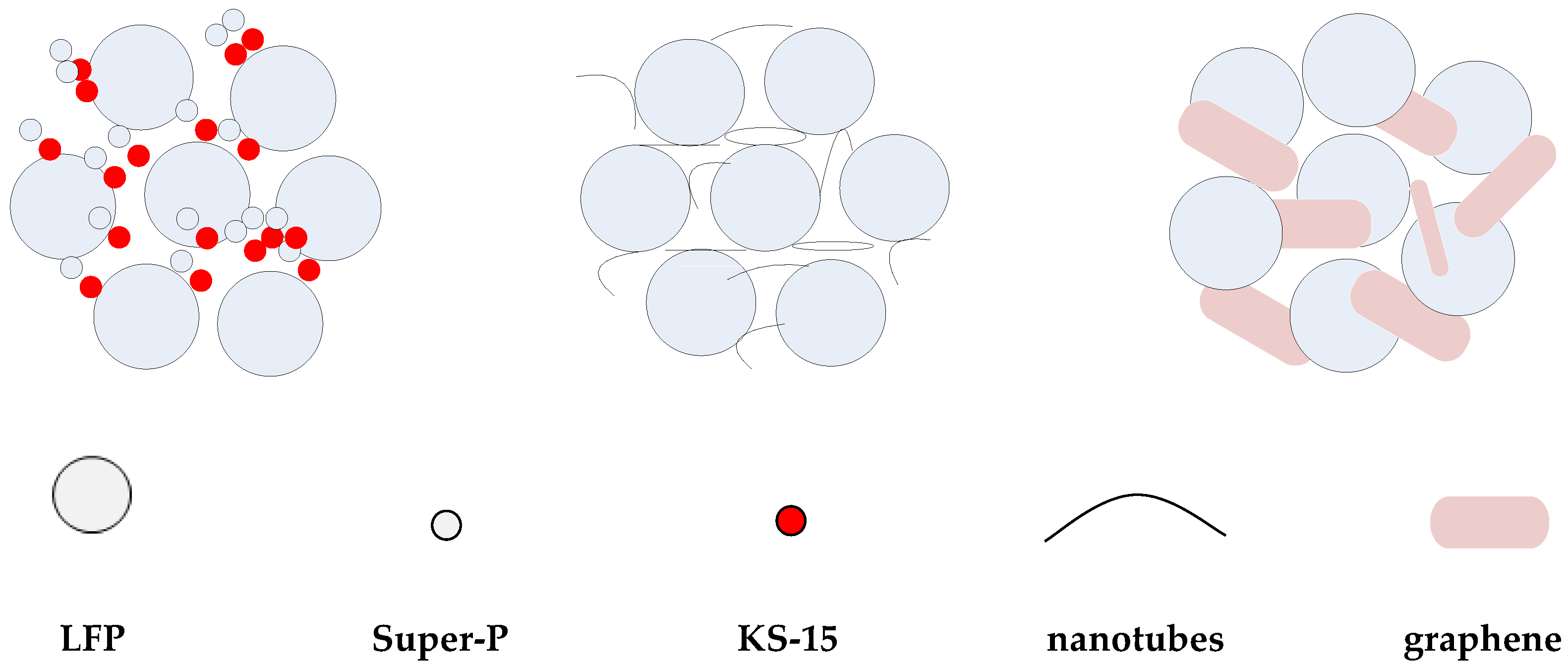
3.2.1. Morphology
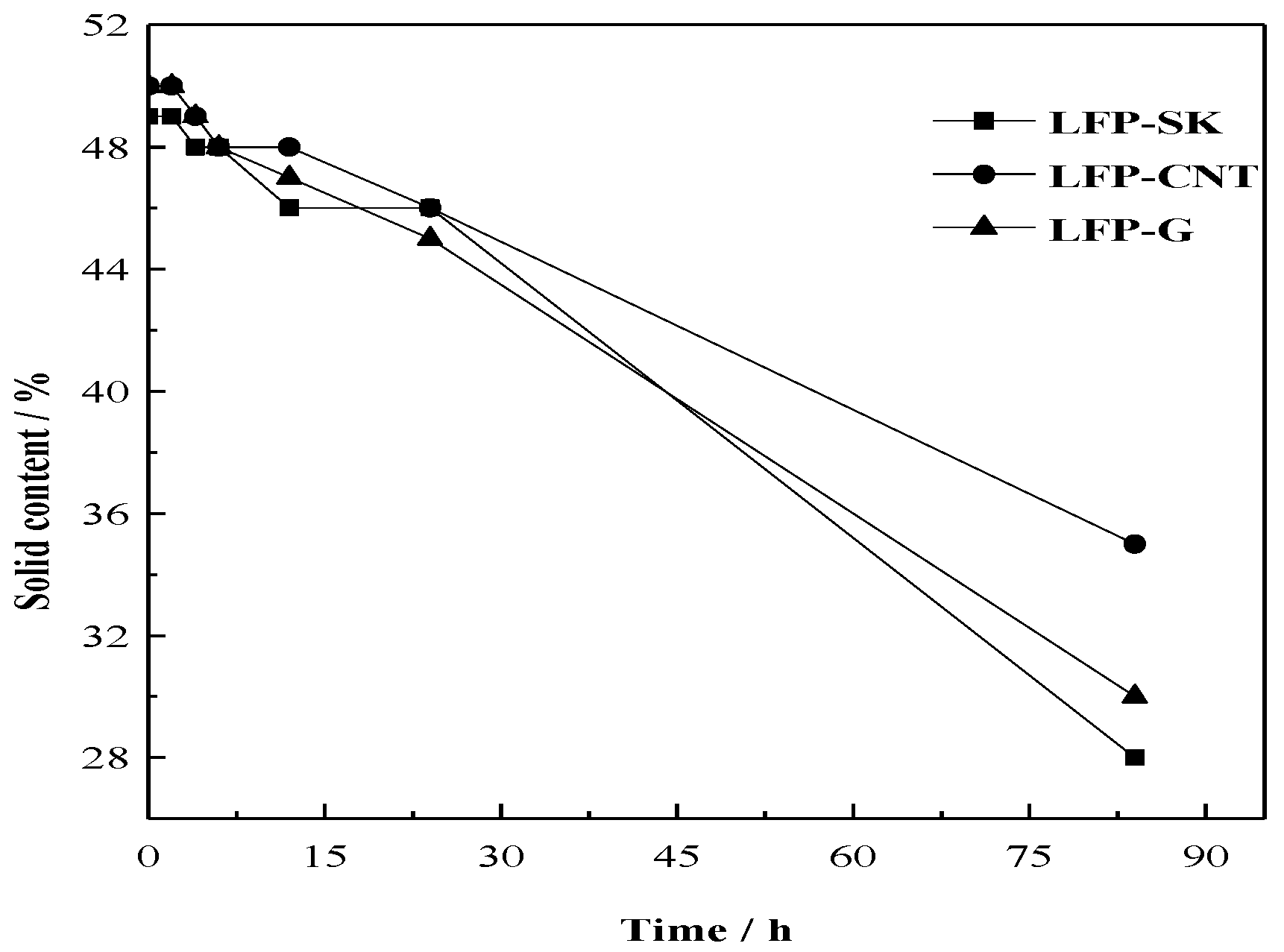
3.2.2. Stability of Solid Content
- m0—the weight of the foil
- m1—the weight of pulp with foil before drying
- m2—the weight of pulp with foil after drying
3.2.3. Cohesiveness Properties
3.2.4. Electrode and Battery Resistance
3.3. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hsieh, C.T.; Pai, C.T.; Chn, Y.F. Preparation of lithium iron phosphate cathode materials with different carbon contents using glucose additive for Li-ion batteries. J. Taiwan Inst. Chem. En. 2014, 45, 1501–1508. [Google Scholar] [CrossRef]
- Jegal, J.P.; Kim, K.B. Carbon nanotube-embedding LiFePO4 as a cathode material for high rate lithium ion batteries. J. Power Sources 2013, 243, 859–864. [Google Scholar] [CrossRef]
- Huang, B.; Zheng, X.D.; Fan, X.P.; Song, G.H.; Lu, M. Enhanced rate performance of nano–micro structured LiFePO/C by improved process for high-power Li-ion batteries. Electrochim. Acta 2011, 56, 4865–4868. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Gong, C.; Xue, Z.; Wen, S.; Ye, Y.; Xie, X. Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J. Power Sources 2016, 318, 93–112. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Vicente, N.; Haro, M.; Cíntora-Juárez, D. LiFePO4, particle conductive composite strategies for improving cathode rate capability. Electrochim. Acta 2015, 163, 323–329. [Google Scholar] [CrossRef]
- Yang, C.C.; Jang, J.H.; Jiang, J.R. Study of electrochemical performances of lithium titanium oxide–coated LiFePO4/C cathode composite at low and high temperatures. Appl. Energy 2016, 162, 1419–1427. [Google Scholar] [CrossRef]
- Tang, H.; Tan, L.; Xu, J. Synthesis and characterization of LiFePO4 coating with aluminum doped zinc oxide. Trans. Nonferrous Met. Soc. China 2012, 23, 451–455. [Google Scholar] [CrossRef]
- Ni, J.F.; Morishita, M.; Kawabe, Y.; Watada, M.; Takeichi, N.; Sakaia, T. Hydrothermal preparation of LiFePO4 nanocrystals mediated by organic acid. J. Power Sources 2010, 195, 2877–2882. [Google Scholar] [CrossRef]
- Starke, B.; Seidlmayer, S.; Jankowsky, S. Influence of particle morphologies of LiFePO4 on water-and solvent-based processing and electrochemical properties. Sustainability Sci. 2017, 9, 888. [Google Scholar] [CrossRef]
- Goktepe, H.; Sahan, H.; Patat, S. Effect of silver and carbon double coating on the electrochemical performance of LiFePO4 cathode material for lithium ion batteries. Int. J. Hydrogen Energy 2016, 41, 9774–9779. [Google Scholar] [CrossRef]
- Prosini, P.P.; Lisi, M.; Zane, D. Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ionics 2002, 148, 45–51. [Google Scholar] [CrossRef]
- Wang, W.Q.; Hao, J.J.; Guo, Z.M. A simple hydrothermal process based on FePO4·2H2O to synthesize spherical LiFePO4/C cathode material. Adv. Mater. Res. 2012, 476–478, 1837–1840. [Google Scholar] [CrossRef]
- Zhou, J.X.; Shen, X.Q.; Jing, M.X. Synthesis and electrochemical performances of spherical LiFePO4 cathode materials for Li-ion batteries. Rare Met. 2006, 25, 19–24. [Google Scholar] [CrossRef]
- Yang, C.C.; Jang, J.H.; Jiang, J.R. Comparison electrochemical performances of spherical LiFePO4/C cathode materials at low and high temperatures. Int. Conf. Appl. Energy 2014, 61, 1402–1409. [Google Scholar] [CrossRef]
- Muller, M.; Pfaffmann, L.; Jaiser, S. Investigation of binder distribution in graphene anodes for lithium-ion batteries. J. Power Sources 2017, 340, 1–5. [Google Scholar] [CrossRef]
- Ilango, P.R.; Gnanamuthu, R.; Jo, Y.N. Design and electrochemical investigation of a novel graphene oxide-silver joint conductive agent on LiFePO4 cathodes in rechargeable lithium-ion batteries. J. Ind. Eng. Chem. 2016, 36, 121–124. [Google Scholar] [CrossRef]
- Liu, W.R.; Guo, Z.Z.; Young, W.S.; Shieh, D.T.; Wu, H.C.; Yang, M.H.; Wu, N.L. Effect of electrode structure on performance of Si anode in Li-ion batteries: Si particle size and conductive additive. J. Power Sources 2005, 140, 139–144. [Google Scholar] [CrossRef]
- Zhu, X.D.; Tian, J.; Le, S.R. Improved electrochemical performance of CuCrO2 anode with CNTs as conductive agent for lithium ion batteries. Mater. Lett. 2013, 97, 113–116. [Google Scholar] [CrossRef]
- Wu, X.L.; Guo, Y.G.; Su, J. Carbon-nanotube-decorated nano-LiFePO4/C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries. Adv. Eng. Mater. 2013, 3, 1155–1160. [Google Scholar] [CrossRef]
- Nair, J.R.; Rius, G.; Jagadale, P. Remarkably stable high power Li-ion battery anodes based on vertically arranged multilayered-graphene. Electrochim. Acta 2015, 182, 500–506. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Feng, W.L.; Li, J. Ultralong cycling stability of carbon-nanotube/LiFePO4 nanocomposites as electrode materials for lithium-ion batteries. Electrochim. Acta 2017, 232, 323–331. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.H.; Wang, T. Dual layer coating strategy utilizing N-doped carbon and reduced graphene oxide for high-performance LiFePO4 cathode material. Electrochim. Acta 2017, 231, 85–93. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Gong, L. A simple route to improve rate performance of LiFePO4/reduced graphene oxide composite cathode by adding Mg2+ via mechanical mixing. J. Power Sources 2017, 347, 29–36. [Google Scholar] [CrossRef]
- Zhu, P.; Yang, Z.Y.; Zeng, P. Homogeneous precipitation synthesis and electrochemical performance of LiFePO4/CNTs/C composites as advanced cathode materials for lithium ion batteries. RSC Adv. 2015, 130, 107293–107298. [Google Scholar] [CrossRef]
- Zhao, N.N.; Li, Y.S.; Zhi, X.K. Effect of Ce3+ doping on the properties of LiFePO4 cathode material. J. Rare Earths 2016, 34, 174–180. [Google Scholar] [CrossRef]
- He, J.C. Effect of carbon source on the particles morphology and carbon structure of LiFePO4/C composites. Adv. Mater. Res. 2014, 968, 53–57. [Google Scholar] [CrossRef]
- Liu, Q.B.; Liao, S.J.; Song, H.Y.; Liang, Z.X. High-performance LiFePO4/C materials: Effect of carbon source on microstructure and performance. J. Power Sources 2012, 211, 52–58. [Google Scholar] [CrossRef]
- Fey, T.K.; Lin, Y.C.; Kao, H.M. Characterization and electrochemical properties of high tap-density LiFePO4/C cathode materials by a combination of carbothermal reduction and molten salt methods. Electrochim. Acta 2012, 80, 41–49. [Google Scholar] [CrossRef]
- Tu, X.F.; Zhou, Y.K.; Tian, X.H. Monodisperse LiFePO4 microspheres embedded with well-dispersed nitrogen-doped carbon nanotubes as high-performance positive electrode material for lithium-ion batteries. Electrochim. Acta 2016, 222, 64–73. [Google Scholar] [CrossRef]


| Samples | Electrode Resistance (Ω) | Battery Internal Resistance (mΩ) |
|---|---|---|
| LFP-SK | 60 | 42 |
| LFP-CNT | 46 | 30 |
| LFP-G | 43 | 35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Sun, J.; An, L.; Wang, X.; Ren, X.; Liang, G. Effect of Conductive Material Morphology on Spherical Lithium Iron Phosphate. Nanomaterials 2018, 8, 904. https://doi.org/10.3390/nano8110904
Wen L, Sun J, An L, Wang X, Ren X, Liang G. Effect of Conductive Material Morphology on Spherical Lithium Iron Phosphate. Nanomaterials. 2018; 8(11):904. https://doi.org/10.3390/nano8110904
Chicago/Turabian StyleWen, Lizhi, Jiachen Sun, Liwei An, Xiaoyan Wang, Xin Ren, and Guangchuan Liang. 2018. "Effect of Conductive Material Morphology on Spherical Lithium Iron Phosphate" Nanomaterials 8, no. 11: 904. https://doi.org/10.3390/nano8110904




