High Performance Acetylene Sensor with Heterostructure Based on WO3 Nanolamellae/Reduced Graphene Oxide (rGO) Nanosheets Operating at Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of WO3/rGO Nanocomposites
2.3. Characterization Techniques
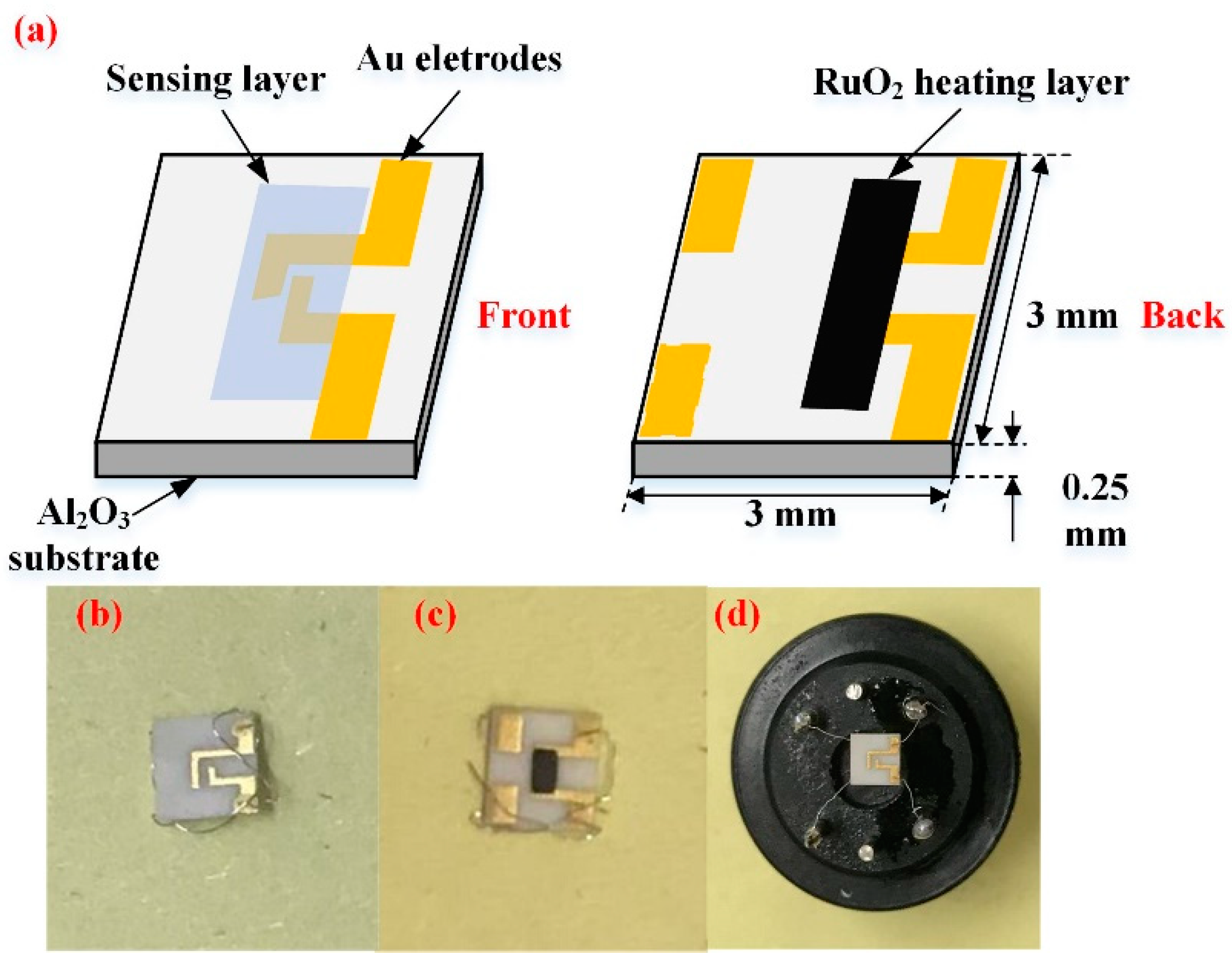
2.4. Fabrication of Planar Gas Sensor
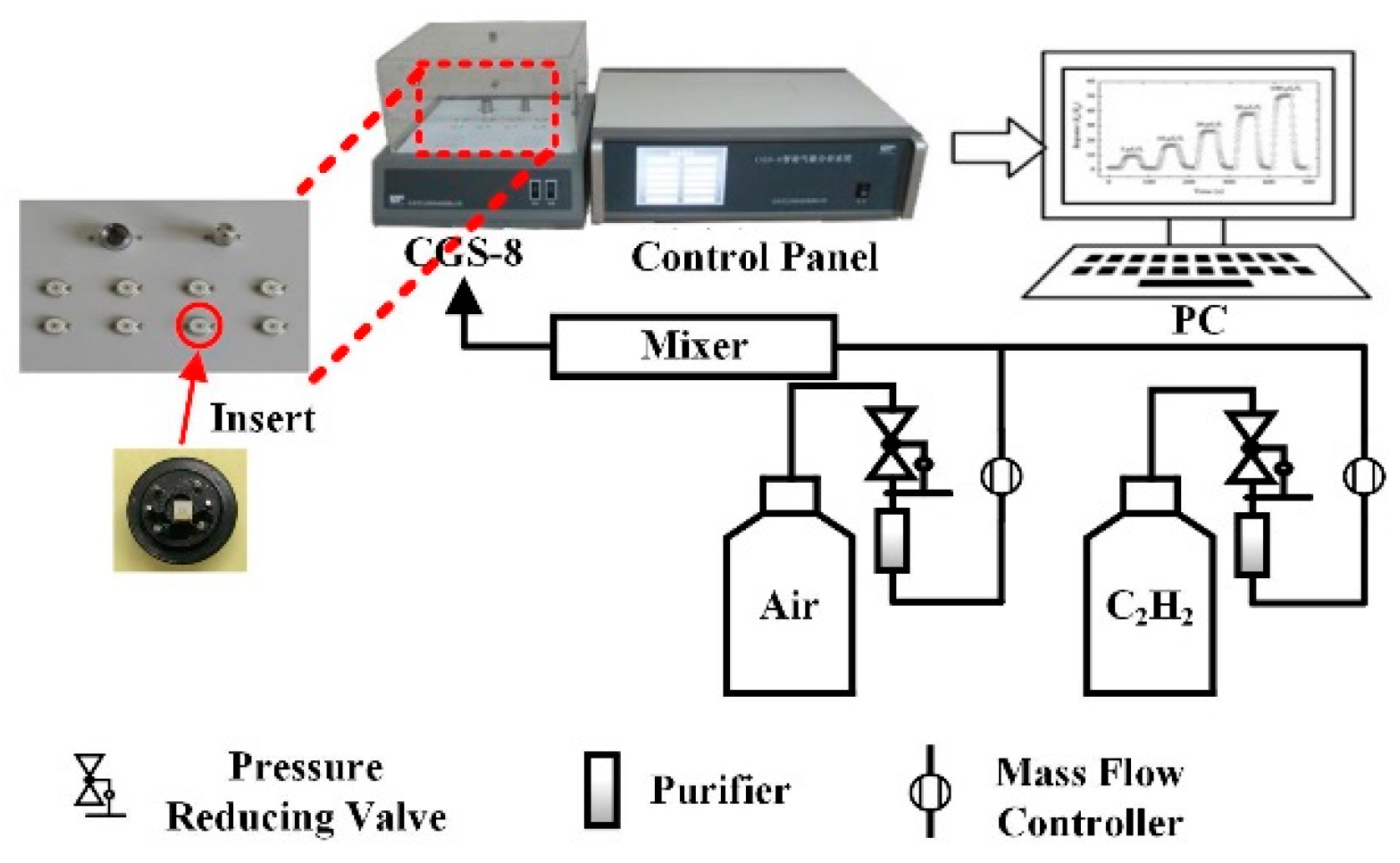
2.5. Gas Sensing Measurements
3. Results and Discussion
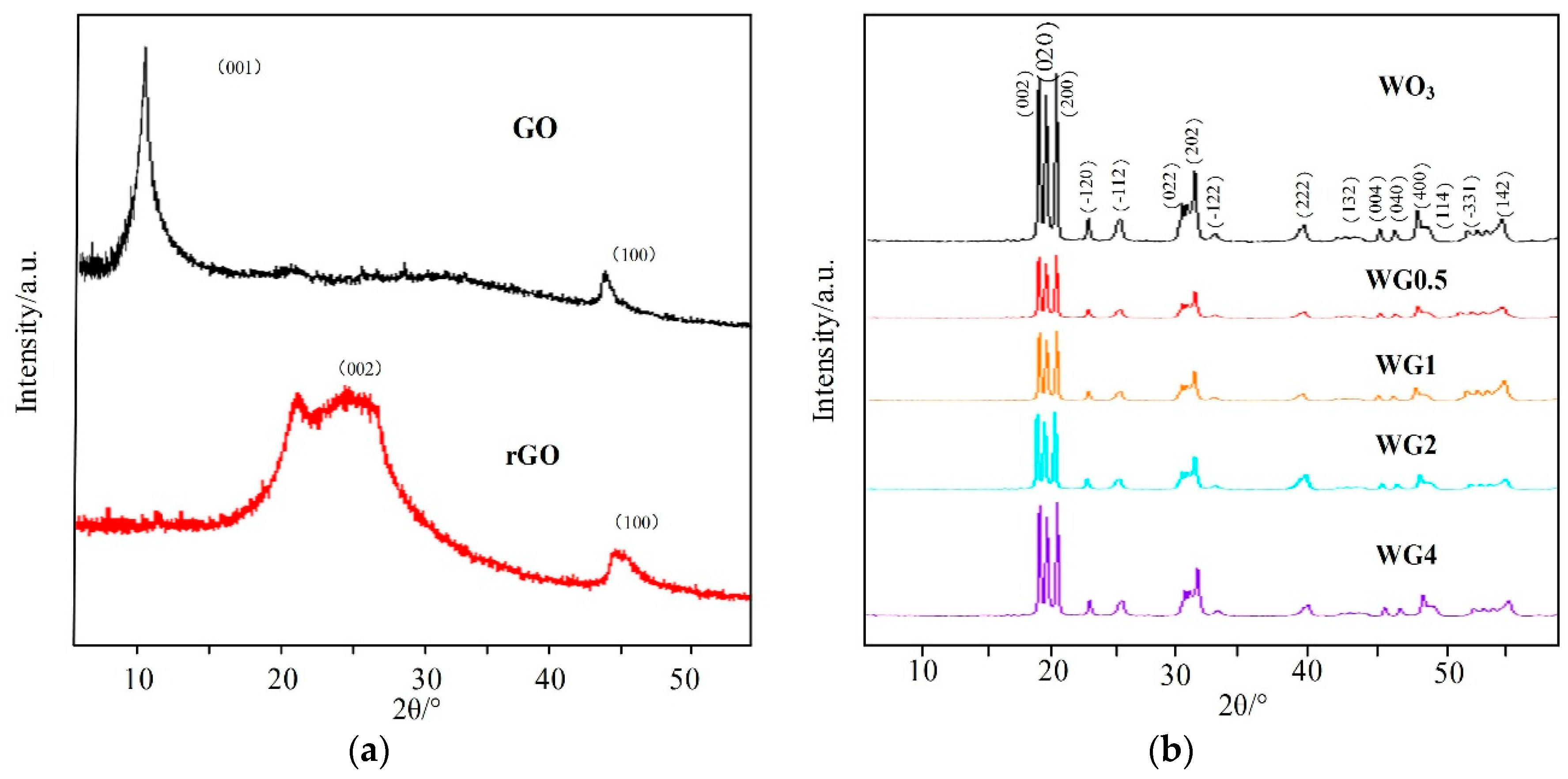
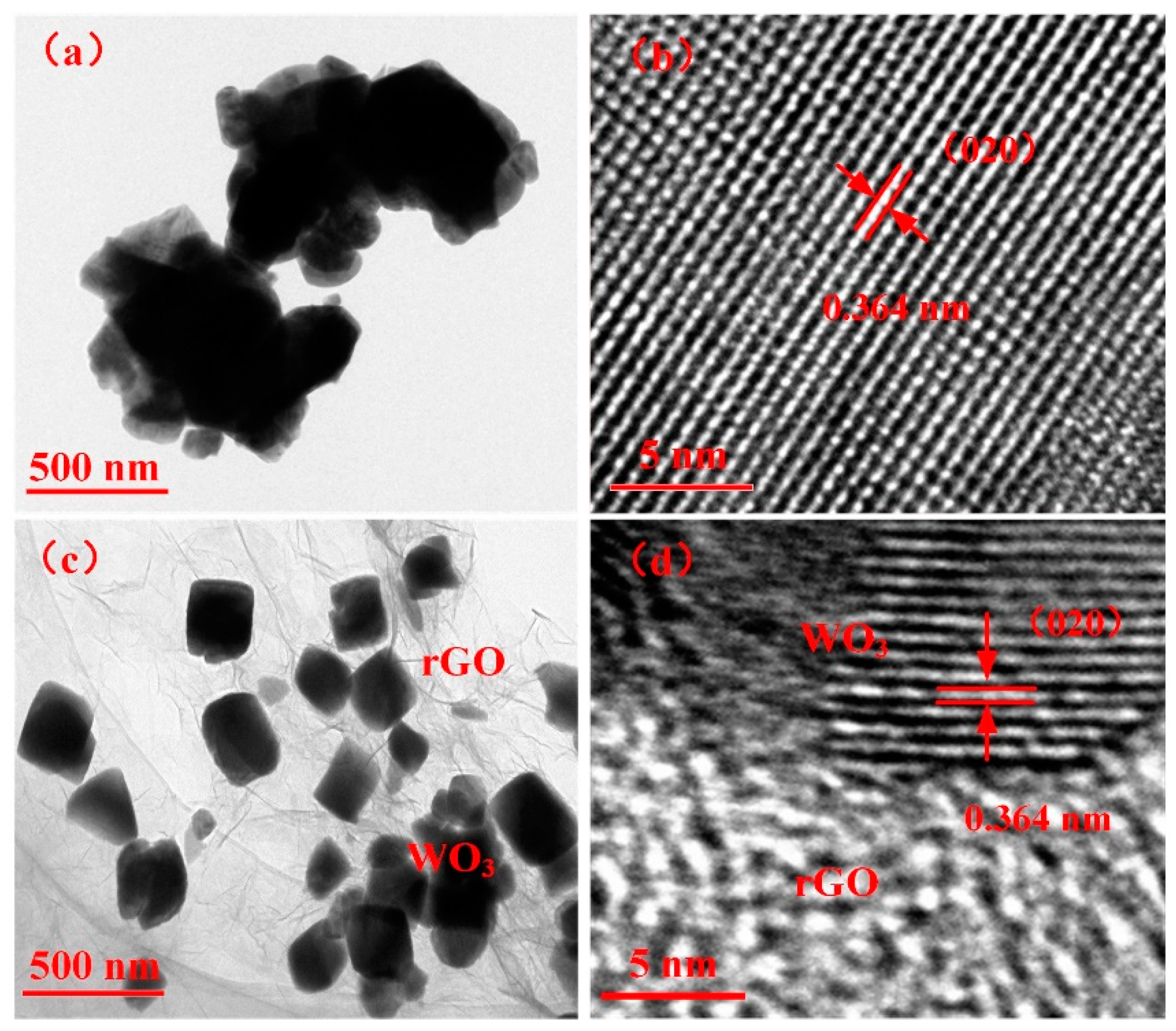
3.1. Structural Characterization
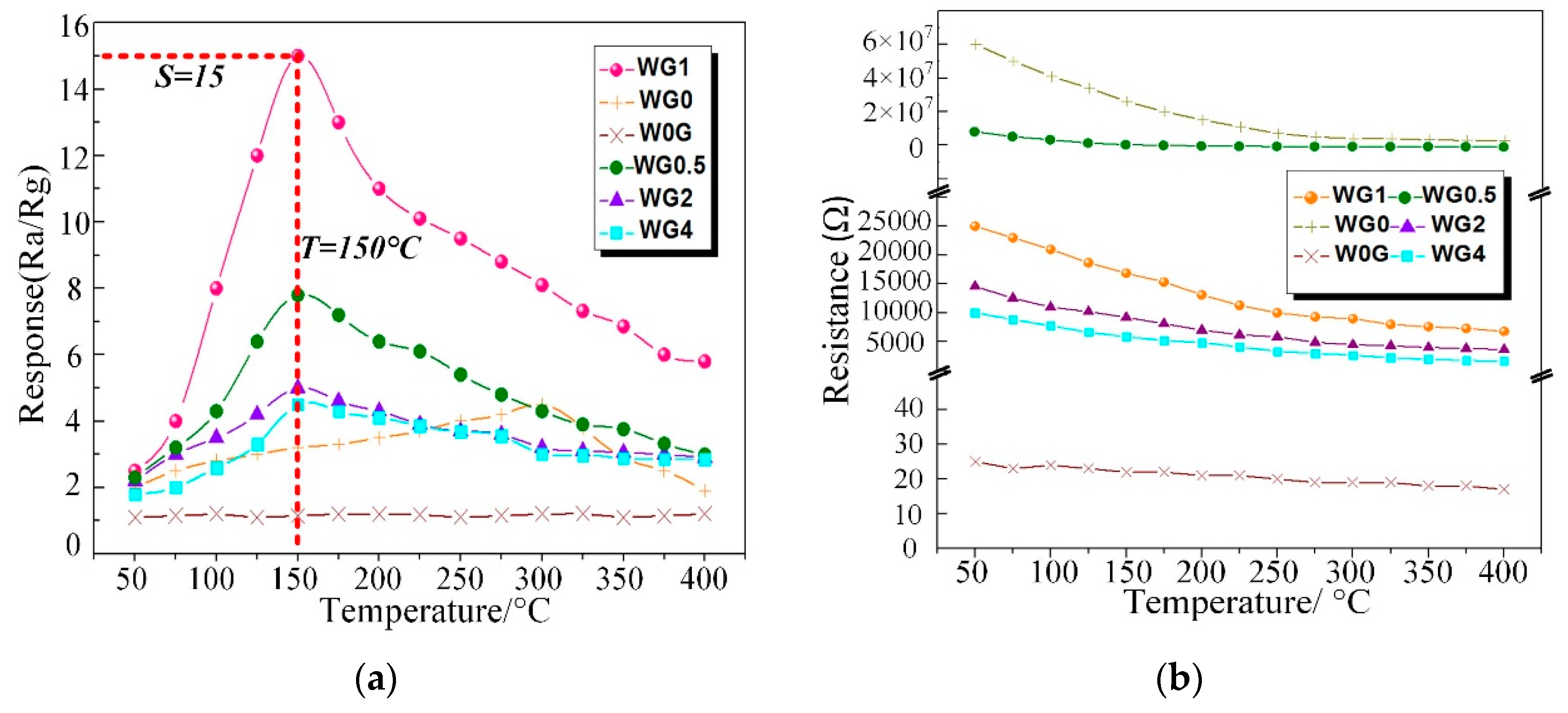
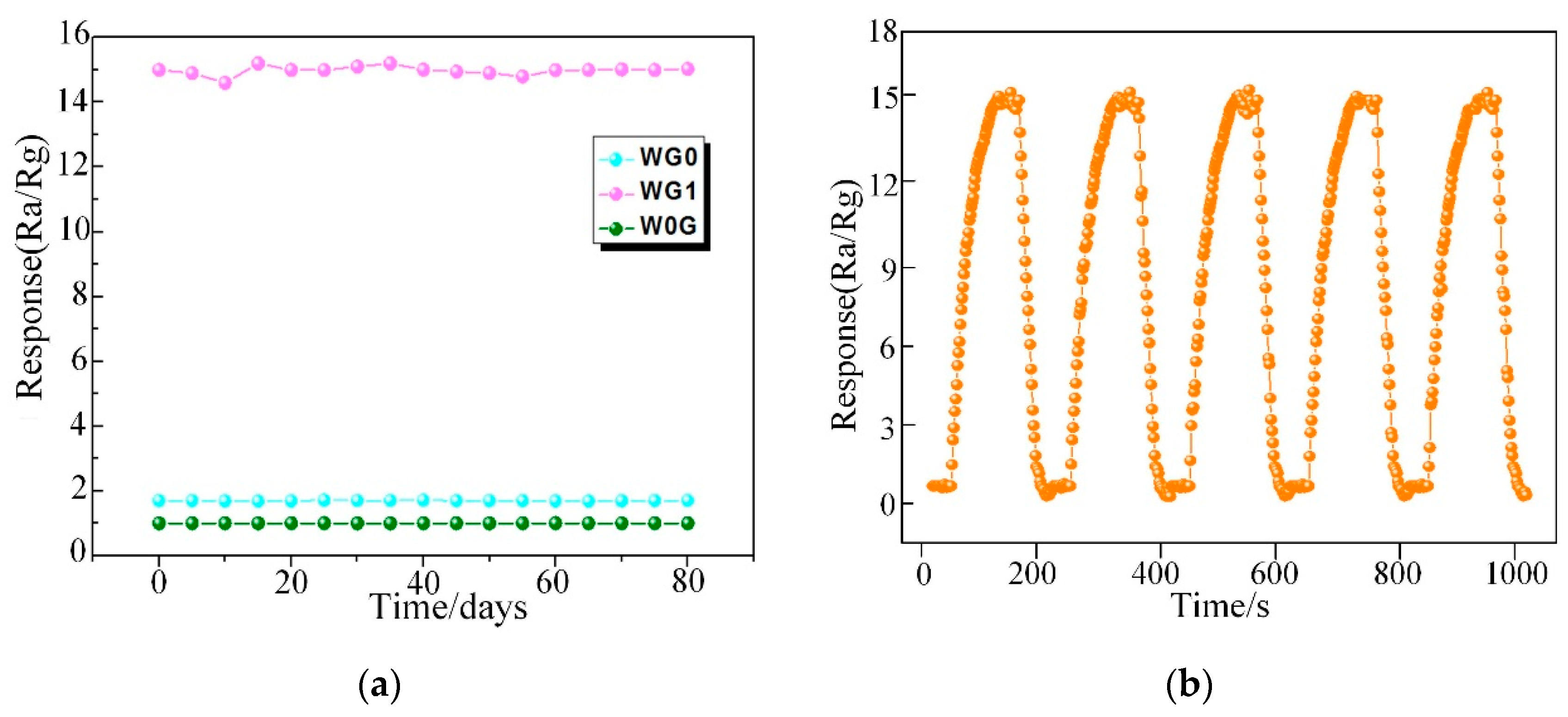
3.2. C2H2 Gas Sensing Properties
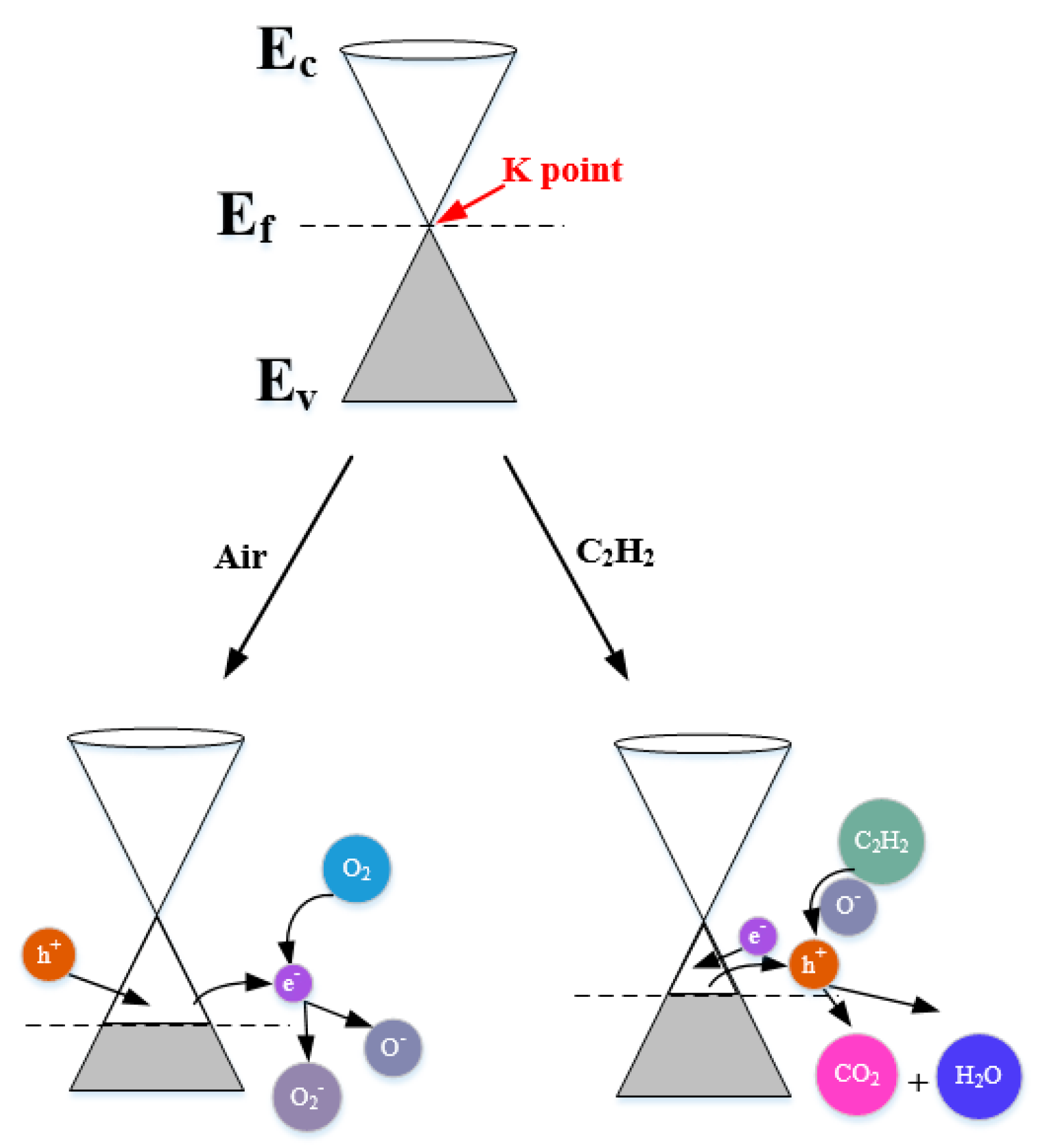
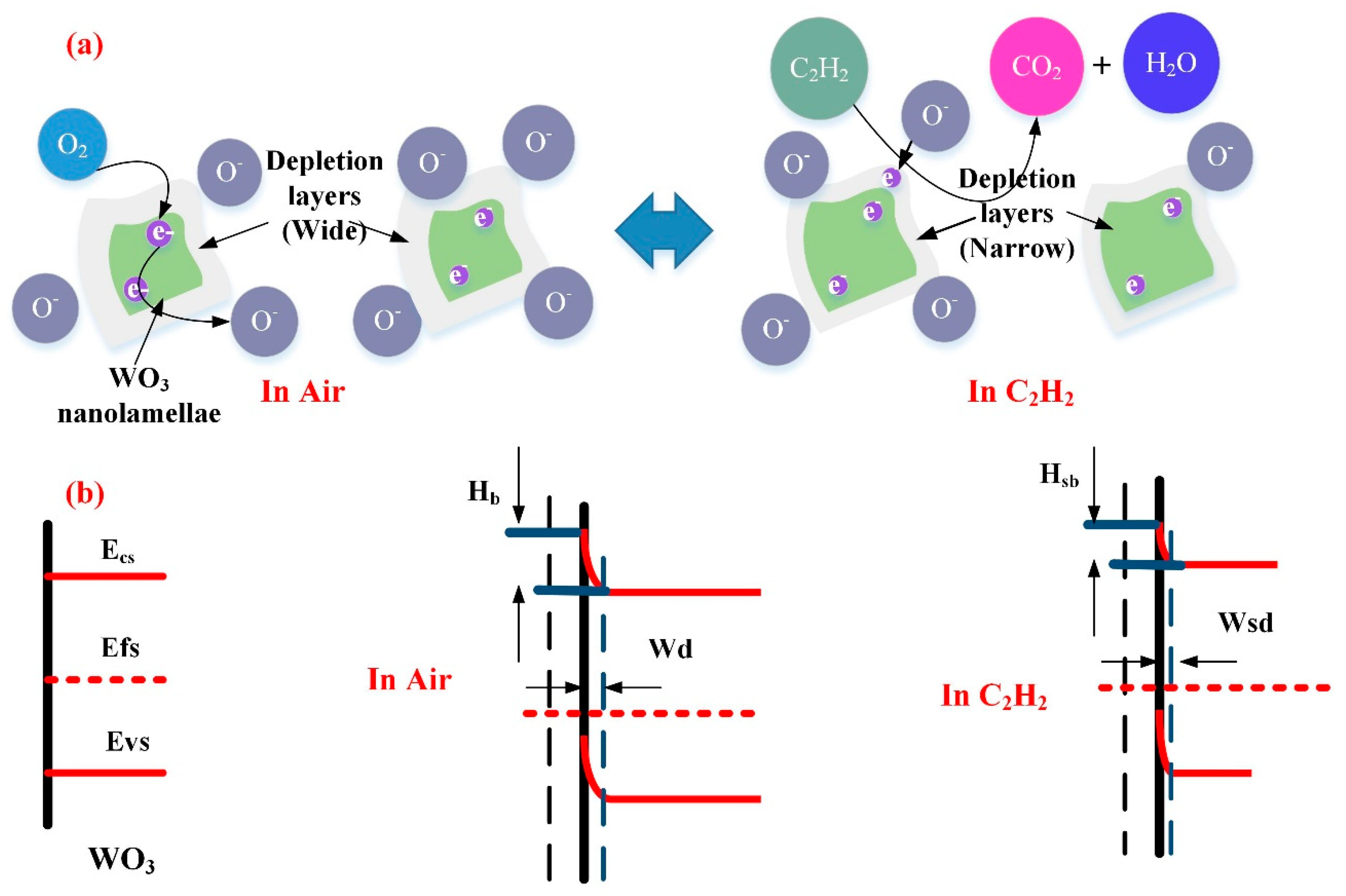
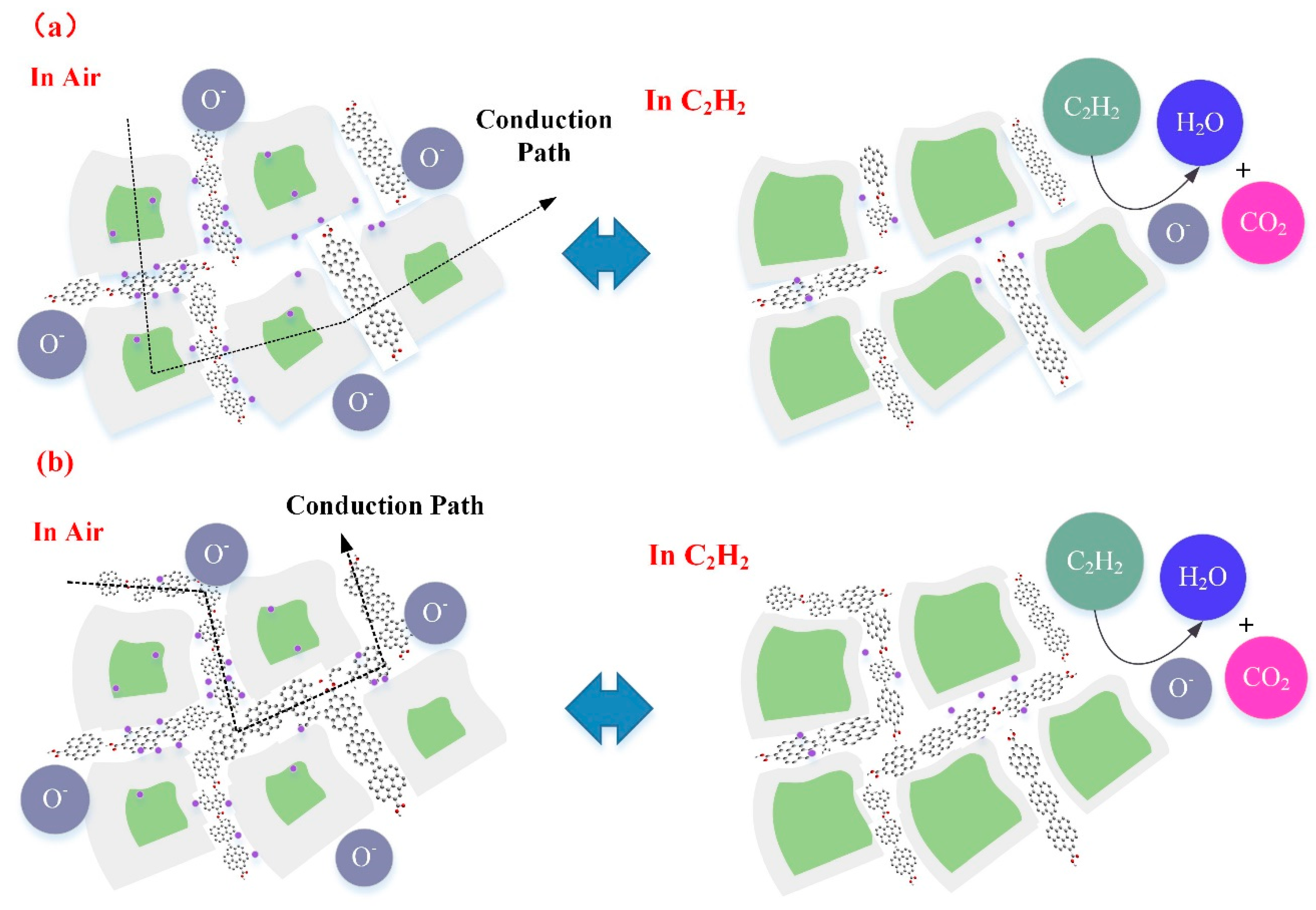
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.Y.; Gong, S.L.; Grzybowski, S. Reliability evaluation method for oil-paper insulation in power transformers. Energies 2011, 4, 1362–1375. [Google Scholar] [CrossRef]
- Illias, H.A.; Liang, W.Z. Identification of transformer fault based on dissolved gas analysis using hybrid support vector machine-modified evolutionary particle swarm optimisation. PLoS ONE 2018, 13, e0191366. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Tang, W.; Wu, Q. Dissolved gas analysis method based on novel feature prioritisation and support vector machine. IET Electr. Power Appl. 2014, 8, 320–328. [Google Scholar] [CrossRef]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.G. Aging of oil-impregnated paper in power transformers. IEEE Trans. Power Deliv. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Wang, Z. Artificial Intelligence Applications in the Diagnosis of Power Transformer Incipient Faults. Available online: https://vtechworks.lib.vt.edu/handle/10919/28594 (accessed on 1 November 2018).
- Sang, Z.X.; Mao, C.X.; Lu, J.M.; Wang, D. Analysis and simulation of fault characteristics of power switch failures in distribution electronic power transformers. Energies 2013, 6, 4246–4268. [Google Scholar] [CrossRef]
- Mineral Oil-Impregnated Electrical Equipment in Service—Guide to the Interpretation of Dissolved and Free Gases Analysis. Available online: https://www.vde-verlag.de/iec-normen/preview-pdf/info_iec60599%7Bed2.1%7Db.pdf (accessed on 1 November 2018).
- Souahlia, S.; Bacha, K.; Chaari, A. MLP neural network-based decision for power transformers fault diagnosis using an improved combination of rogers and doernenburg ratios DGA. Int. J. Electr. Power Energy Syst. 2012, 43, 1346–1353. [Google Scholar] [CrossRef]
- Coward, H.F.; Jones, G.W. Limits of inflammability of gases and vapors. J. Franklin Inst. 1928, 203, 161. [Google Scholar] [CrossRef]
- Cashdollar, K.L.; Zlochower, I.A.; Green, G.M.; Thomas, R.A.; Hertzberg, M. Flammability of methane, propane, and hydrogen gases. J. Loss Prev. Process Ind. 2000, 13, 327–340. [Google Scholar] [CrossRef]
- Wang, L.W.; Kang, Y.F.; Liu, X.H.; Zhang, S.M.; Huang, W.P.; Wang, S.R. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Meng, D.; Yamazaki, T.; Shen, Y.; Liu, Z.; Kikuta, T. Preparation of WO3 nanoparticles and application to NO2 sensor. Appl. Surf. Sci. 2009, 256, 1050–1053. [Google Scholar] [CrossRef]
- Zou, X.; Li, G.; Wang, P.; Su, J.; Zhao, J.; Zhou, L.; Wang, Y.N.; Chen, J. A precursor route to single-crystalline WO3 nano-plates with an uneven surface and enhanced sensing properties. Dalton Trans. 2012, 41, 9773–9780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Yang, J.; Guo, W.; Zhu, L.; Zheng, W. Temperature and acidity effects on WO3 nanostructures and gas-sensing properties of WO3 nanoplates. Mater. Res. Bull. 2014, 57, 260–267. [Google Scholar] [CrossRef]
- Wei, S.; Xing, Y.; Li, Y.; Zhao, Y.; Du, W.; Zhou, M. Preparation and gas sensing properties of flower-like WO3 hierarchical architecture. Vacuum 2016, 129, 13–19. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Fardindoost, S.; Zad, A.I. Fabrication of Pd Doped WO3 Nanofiber as Hydrogen Sensor. Polymers 2013, 5, 45–55. [Google Scholar] [CrossRef]
- Hu, T.; Wang, D.; Wang, M.; Li, Z.; Yang, M. Miniature Hydrogen Sensor Based on Fiber Inner Cavity and Pt-doped WO3 Coating. IEEE Photonics Technol. Lett. 2014, 26, 1458–1461. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, K.; Kohli, N.; Kaur, A.; Singh, R.C. Temperature dependent selective detection of hydrogen and acetone using Pd doped WO3/Reduced graphene oxide nanocomposite. Chem. Phys. Lett. 2018, 701, 115–125. [Google Scholar] [CrossRef]
- Kuar, J.; Anand, K.; Kaur, A.; Singh, R.C. Sensitive and selective acetone sensor based on Gd doped WO3/reduced graphene oxide nanocomposite. Sens. Actuators B Chem. 2017, 258, 1022–1035. [Google Scholar] [CrossRef]
- Shi, J.; Chenga, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Q.; Gao, T.; Su, X.; Wan, Fu. Pd-doped SnO2-based sensor detecting characteristic fault hydrocarbon gases in transformer oil. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Zhang, H. Hierarchically porous WO3 microstructures with networks for acetylene sensing application. Mater. Lett. 2018, 214, 198–201. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, Y.; Koep, E.; Liu, M. A highly sensitive and fast-responding SnO2 sensor fabricated by combustion chemical vapor deposition. Chem. Mater. 2005, 17, 3997–4000. [Google Scholar] [CrossRef]
- Rossi, C.; Conto, T.D.; Estève, D.; Larangot, B. Design, fabrication and modelling of MEMS-based microthrusters for space application. Smart Mater. Struct. 2001, 10, 1156. [Google Scholar] [CrossRef]
- Matsui, M. Thermal Stability of Au Thin Film Deposited on Al2O3 Substrate with RuO2 Adhesion Layer. J. Ceram. Soc. Jpn. 2001, 109, 574–575. [Google Scholar] [CrossRef]
- Norris, B.J.; Anderson, J.; Wager, J.F.; Keszler, D.A. Spin-coated zinc oxide transparent transistors. J. Phys. D Appl. Phys. 2003, 36, L105. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, D.; Du, Z.; Yin, X.; Chen, L.; Li, Q.; Wang, Y.; Wang, T. Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J. Mater. Chem. 2011, 21, 1673–1676. [Google Scholar] [CrossRef]
- Chen, X.; Kalenczuk, R.J.; Wajda, A.; Łapczuk, J.; Kurzewski, M.; Drozdzik, M.; Chu, P.K.; Palen, E.B. Synthesis, disper-sion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colliods Surf. B 2012, 89, 79–85. [Google Scholar]
- Upadhyay, S.B.; Mishra, R.K.; Sahay, P.P. Enhanced acetone response in co-precipitated WO3 nanostructures upon indium doping. Sens. Actuators B Chem. 2015, 209, 368–376. [Google Scholar] [CrossRef]
- Tang, L.; Wan, Y.; Yan, D.; Pei, Y.; Zhao, L.; Li, Y.; Wu, L.; Jiang, J.; Lai, G. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Zhu, G.; Zhou, H.; Yuan, A. Reduced graphene oxide/nickel nanocomposites: Facile synthesis, magnetic and catalytic properties. J. Mater. Chem. 2012, 22, 3471–3477. [Google Scholar] [CrossRef]
- Garciasanchez, R.F.; Ahmido, T.; Casimir, D.; Baliga, S.; Misra, P. Thermal Effects Associated with the Raman Spectroscopy of WO3 Gas-Sensor Materials. J. Phys. Chem. A 2013, 117, 13825–13831. [Google Scholar] [CrossRef] [PubMed]
- Park, O.; Hahm, M.G.; Lee, S.; Joh, H.; Na, S.; Vajtai, R.; Lee, J.H.; Ku, B.C.; Ajayan, P.M. In Situ Synthesis of Thermochemically Reduced Graphene Oxide Conducting Nanocomposites. Nano. Lett. 2012, 12, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J. Percolation effect of reduced graphene oxide (rGO) on ammonia sensing of rGO-SnO2 composite based sensor. Sens. Actuator B 2017, 243, 1115–1126. [Google Scholar] [CrossRef]
- Dutta, D.; Wood, B.C.; Bhide, S.Y.; Ayappa, K.G.; Narasimhan, S. Enhanced Gas Adsorption on Graphitic Substrates via Defects and Local Curvature: A Density Functional Theory Study. J. Phys. Chem. C 2014, 118, 7741–7750. [Google Scholar] [CrossRef]
- Díazreyes, J.; Dorantes-García, V.; Pérez-Benítez, A.; Balderas-López, J.A. Obtaining of films of tungsten trioxide (WO3) by resistive heating of a tungsten filament. Superficies Y Vacío 2008, 21, 1665–3521. [Google Scholar]
- Hardcastle, F.D.; Wachs, I.E. Determination of the molecular structures of tungstates by Raman spectroscopy. J. Raman Spectrosc. 1995, 26, 397–405. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Lo, S.S.; Huang, D. Morphological variation and raman spectroscopy of zno hollow microspheres prepared by a chemical colloidal process. J. Surf. Colloids 2010, 26, 6762–6766. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.I.; Abdel-Khalik, M.; Habashy, G.M. Acid-leaching and consequent pore structure and bleaching capacity modifications of egyptian clays. Colloids Surf. 1986, 17, 241–249. [Google Scholar] [CrossRef]
- Shojaee, M.; Nasresfahani, S.; Sheikhi, M.H. Hydrothermally synthesized Pd-loaded SnO2/partiallyreduced graphene oxide nanocomposite for effective detection of carbon monoxide at room temperature. Sens. Actuators B Chem. 2018, 254, 457–467. [Google Scholar] [CrossRef]
- Anand, K.; Singh, M.P.; Singh, O.; Kohli, N.; Singh, R.C. Optical and thermal properties of precursor-controlled graphene–zinc nanocomposites. Mater. Sci. Semicon. Proc. 2013, 16, 1706–1712. [Google Scholar] [CrossRef]
- Lv, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, O.; Xie, Z.; Zheng, L. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar]
- Penner, R.M. Hybrid electrochemical/chemical synthesis of quantum dots. Acc. Chem. Res. 2000, 33, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Guo, M.; Zhang, M. Hydrothermal preparation and optical properties of orientation-controlled WO3 nanorod arrays on ITO substrates. Cryst. Eng. Commun. 2013, 15, 277–284. [Google Scholar]
- Lupan, O.; Emelchenko, G.A.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Tiginyanu, I.M.; Chow, L.; Ono, L.K.; Cuenya, B.R.; Heinrich, H. Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide–properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, Z.; Liu, B. Solvothermal synthesis and characterization of a novel reduced graphene oxide (RGO)/BiVO4/SiO2, nanocomposites. Mater. Lett. 2016, 185, 32–35. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Sun, D.; Ren, N.; Yu, J.; Yang, R.; Liu, H. Microwave-assisted hydrothermal synthesis of Sn3O4, nanosheet/rgo planar heterostructure for efficient photocatalytic hydrogen generation. Appl. Catal. B 2018, 227, 470–476. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, J.; Liu, G.; Wang, L. Microwave-assisted synthesis and surface decoration of lifepo 4, hexagonal nanoplates for lithium-ion batteries with excellent electrochemical performance. J. Mater. Sci. 2017, 52, 1590–1602. [Google Scholar] [CrossRef]
- Zang, L.Y.; Misra, H.P. Epr kinetic studies of superoxide radicals generated during the autoxidation of 1-methyl-4-phenyl-2,3-dihydropyridinium, a bioactivated intermediate of parkinsonian-inducing neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Biol. Chem. 1992, 267, 23601. [Google Scholar] [PubMed]
- Chen, Z.; Sun, P.; Fan, B.; Zhang, Z.; Fang, X. In situ template-free ion-exchange process to prepare visible-light-active g-C3N4/Nis hybrid photocatalysts with enhanced hydrogen evolution activity. J. Phys. Chem. C 2014, 118, 7801–7807. [Google Scholar] [CrossRef]
- Jin, L.; Chen, W.; Zhang, H.; Xiao, G.; Yu, C.; Zhou, Q. Characterization of Reduced Graphene Oxide (rGO)-Loaded SnO2 Nanocomposite and Applications in C2H2 Gas Detection. Appl. Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Lee, K.W.; Chung, G.S. Acetylene gas sensing properties of an Ag-loaded hierarchical ZnO nanostructure-decorated reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 216, 33–40. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Chung, G.S. Synthesis of highly dispersed ZnO nanoparticles on graphene surface and their acetylene sensing properties. Sens. Actuators B Chem. 2014, 205, 338–344. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Phanichphant, S. Highly selective environmental sensors based on flame-spray-made SnO2 nanoparticles. Sens. Actuators B Chem. 2012, 163, 51–60. [Google Scholar] [CrossRef]
- Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Phanichphant, S. Acetylene sensor based on Pt/ZnO thick films as prepared by flame spray pyrolysis. Sens. Actuators B Chem. 2011, 152, 155–161. [Google Scholar] [CrossRef]
- Lee, K.W.; Uddin, A.S.M.I.; Phan, D.T.; Chung, G.S. Fabrication of low-temperature acetylene gas sensor based on Ag nanoparticles-loaded hierarchical ZnO nanostructures. Electron. Lett. 2015, 51, 572–574. [Google Scholar] [CrossRef]
- Tammanoon, N.; Wisitsoraat, A.; Sriprachuabwong, C.; Phokharatkul, D.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Ultrasensitive NO2 sensor based on ohmic metal–semiconductor interfaces of electrolytically exfoliated graphene/flame-spray-made SnO2 nanoparticles composite operating at low temperatures. ACS Appl. Mater. Interfaces 2015, 7, 24338–24352. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Dutta, N.K.; Choudhury, N.R. Work function engineering of graphene. Nanomaterials 2014, 4, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Quang, P.L.; Cuong, N.D.; Hoa, T.T.; Long, H.T.; Chu, M.H.; Le, D.T.T.; Hieu, N.Y. Simple post-synthesis of mesoporous p-type Co3O4, nanochains for enhanced H2S gas sensing performance. Sens. Actuators B Chem. 2018, 270, 158–166. [Google Scholar] [CrossRef]
- Acharya, S.; Bangera, K.V.; Shivakumar, G.K. Conduction mechanism in n-CdSe/p-ZnTe heterojunction. J. Electron. Mater. 2016, 45, 3324–3331. [Google Scholar] [CrossRef]
- Chen, W.G.; Gao, T.Y.; Li, Q.Z.; Gan, H.I. Enhanced gas sensing properties of flower-like ZnO nanostructure to acetylene. Mater. Process. Rep. 2014, 30, 96–100. [Google Scholar] [CrossRef]
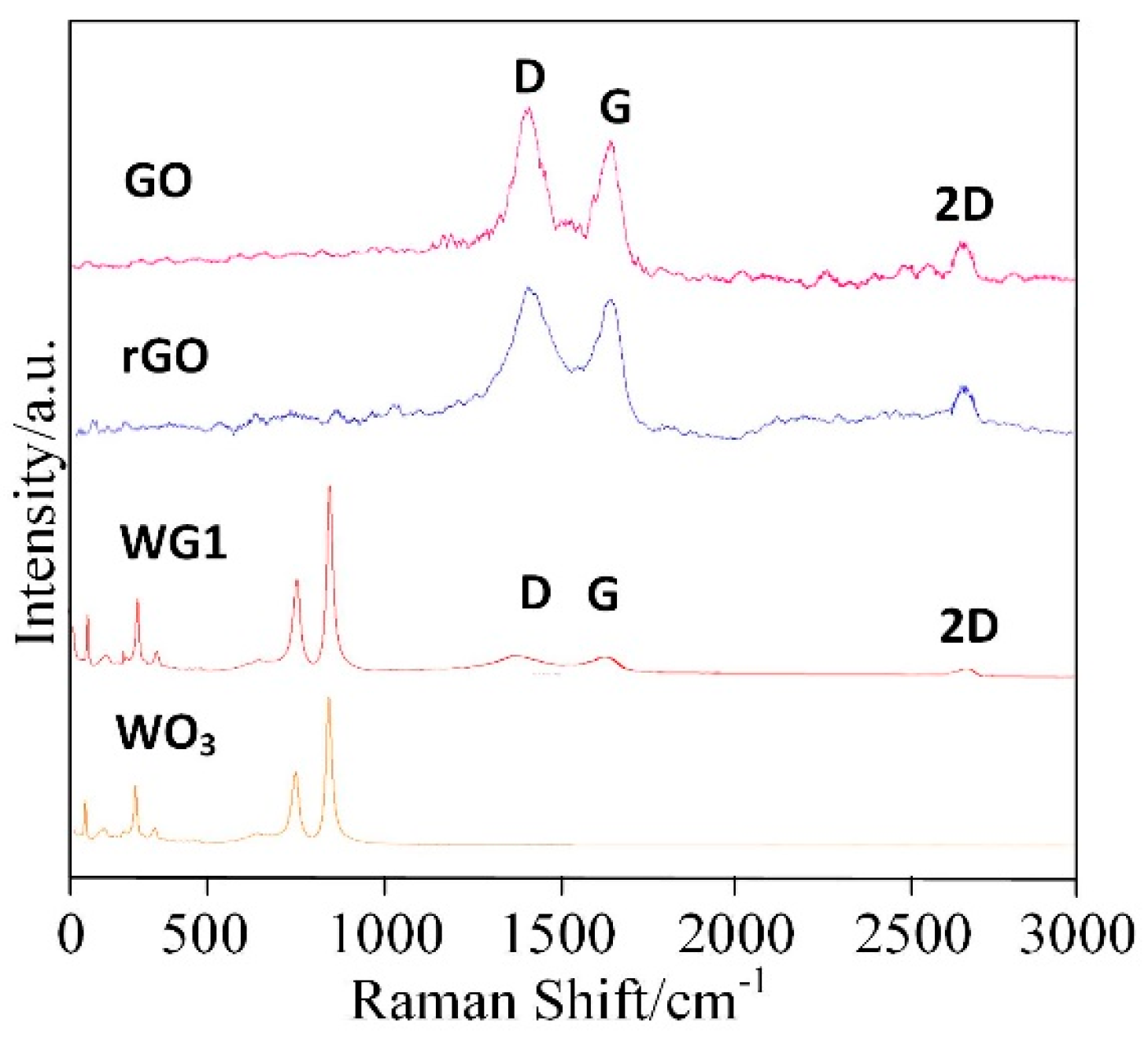
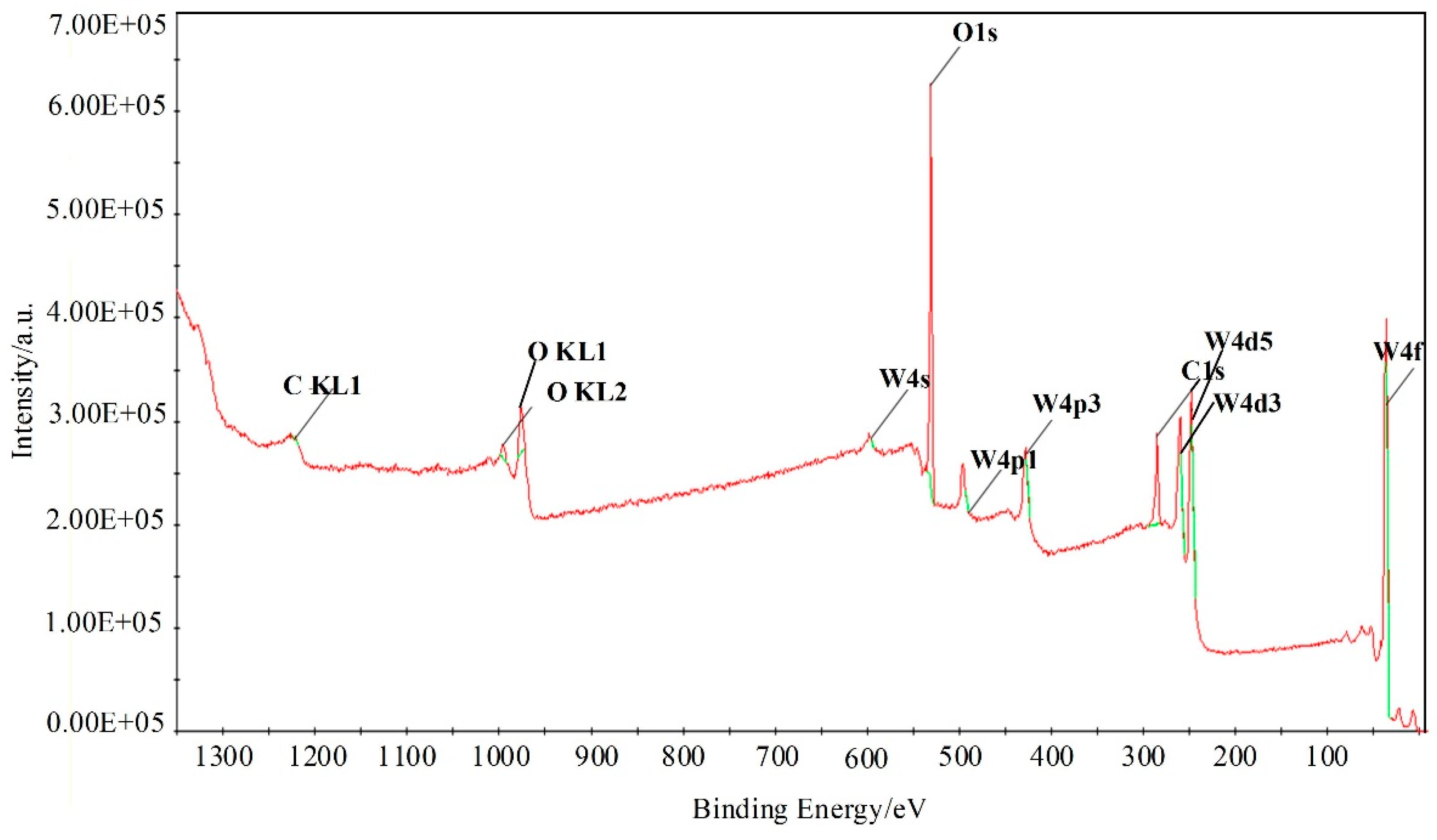
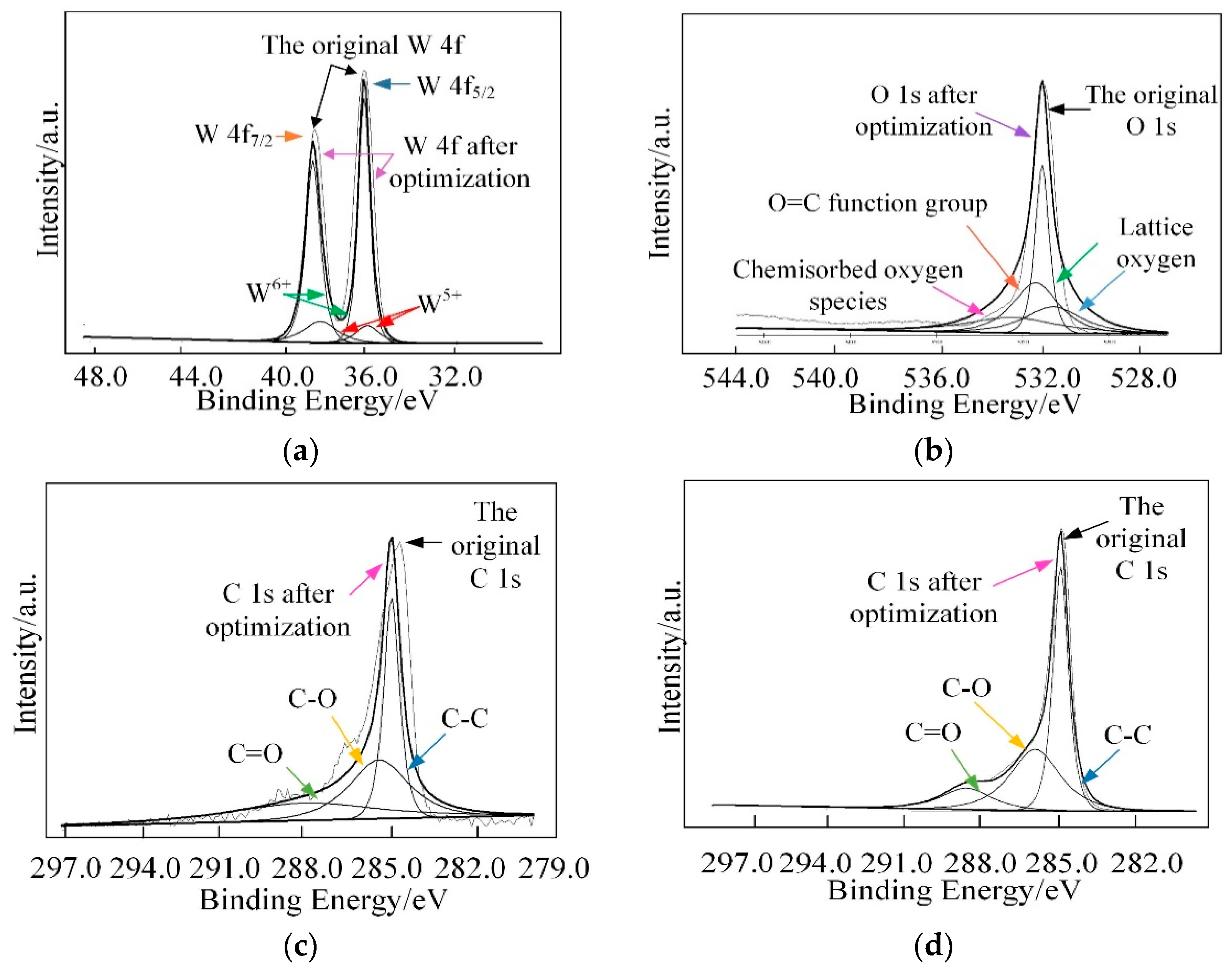

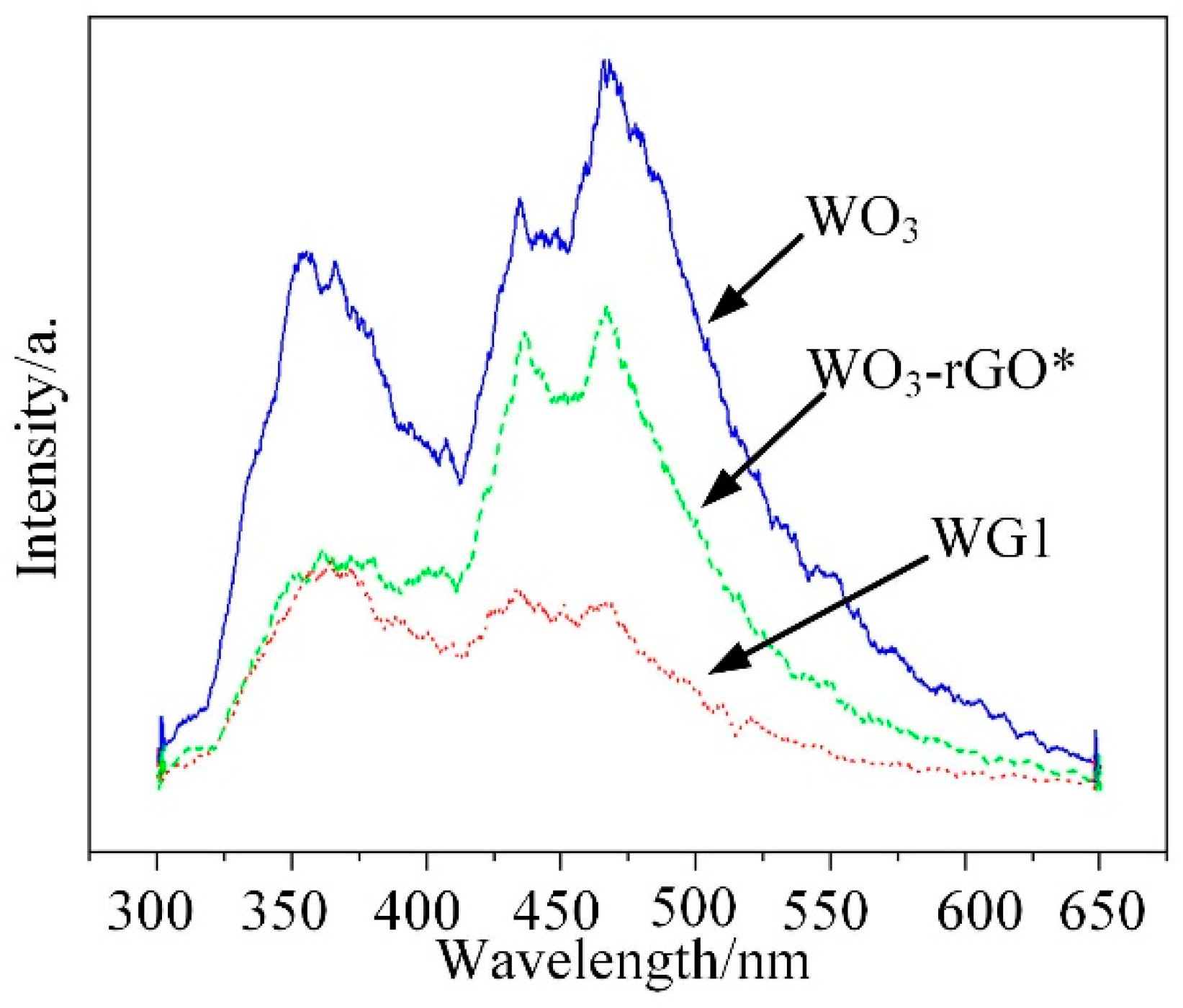


| Ref | Materials | Temperature/°C | Measuring Range/ppm | Response | τResponse/s | τRecovery/s |
|---|---|---|---|---|---|---|
| [22] | WO3 nanorod | 300 | 200 | 35 (200 ppm) | 10 | 9 |
| [22] | WO3 nanowire | 300 | 200 | 60 (200 ppm) | 6 | 7 |
| [57] | ZnO NPs 1 | >300 | 30–1000 | 6.4 (100 ppm) | >2 min | >3 min |
| [58] | Ag-ZnO NPs-rGO | 150 | 1–1000 | 21.2 (100 ppm) | 25 | 80 |
| [57] | ZnO Hrc 2 | >300 | 30–1000 | 11.2 (100 ppm) | >2 min | >6 min |
| [58] | Ag-ZnO Hrc-rGO | 200 | 3–1000 | 12.3 (100 ppm) | 43 | 100 |
| [59] | Pure SnO2 | 300 | 200–10,000 | 6.3 (10,000 ppm) | 34 | >10 min |
| [60] | Pt/ZnO | 300 | 5–10,000 | 1.1 (50 ppm) | N/A | N/A |
| [61] | Ag-ZnO Hrc | 200 | 1–1000 | 57 (50 ppm) | N/A | N/A |
| Our | WG1 | 150 | 1–500 | 15 (50 ppm) | 52 | 21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Chen, W.; Jin, L.; Cui, F.; Song, Z.; Zhu, C. High Performance Acetylene Sensor with Heterostructure Based on WO3 Nanolamellae/Reduced Graphene Oxide (rGO) Nanosheets Operating at Low Temperature. Nanomaterials 2018, 8, 909. https://doi.org/10.3390/nano8110909
Jiang Z, Chen W, Jin L, Cui F, Song Z, Zhu C. High Performance Acetylene Sensor with Heterostructure Based on WO3 Nanolamellae/Reduced Graphene Oxide (rGO) Nanosheets Operating at Low Temperature. Nanomaterials. 2018; 8(11):909. https://doi.org/10.3390/nano8110909
Chicago/Turabian StyleJiang, Zikai, Weigen Chen, Lingfeng Jin, Fang Cui, Zihao Song, and Chengzhi Zhu. 2018. "High Performance Acetylene Sensor with Heterostructure Based on WO3 Nanolamellae/Reduced Graphene Oxide (rGO) Nanosheets Operating at Low Temperature" Nanomaterials 8, no. 11: 909. https://doi.org/10.3390/nano8110909
APA StyleJiang, Z., Chen, W., Jin, L., Cui, F., Song, Z., & Zhu, C. (2018). High Performance Acetylene Sensor with Heterostructure Based on WO3 Nanolamellae/Reduced Graphene Oxide (rGO) Nanosheets Operating at Low Temperature. Nanomaterials, 8(11), 909. https://doi.org/10.3390/nano8110909





