Influence of Mono- and Bimetallic PtOx, PdOx, PtPdOx Clusters on CO Sensing by SnO2 Based Gas Sensors
Abstract
:1. Introduction
2. Results and Discussion
- (i)
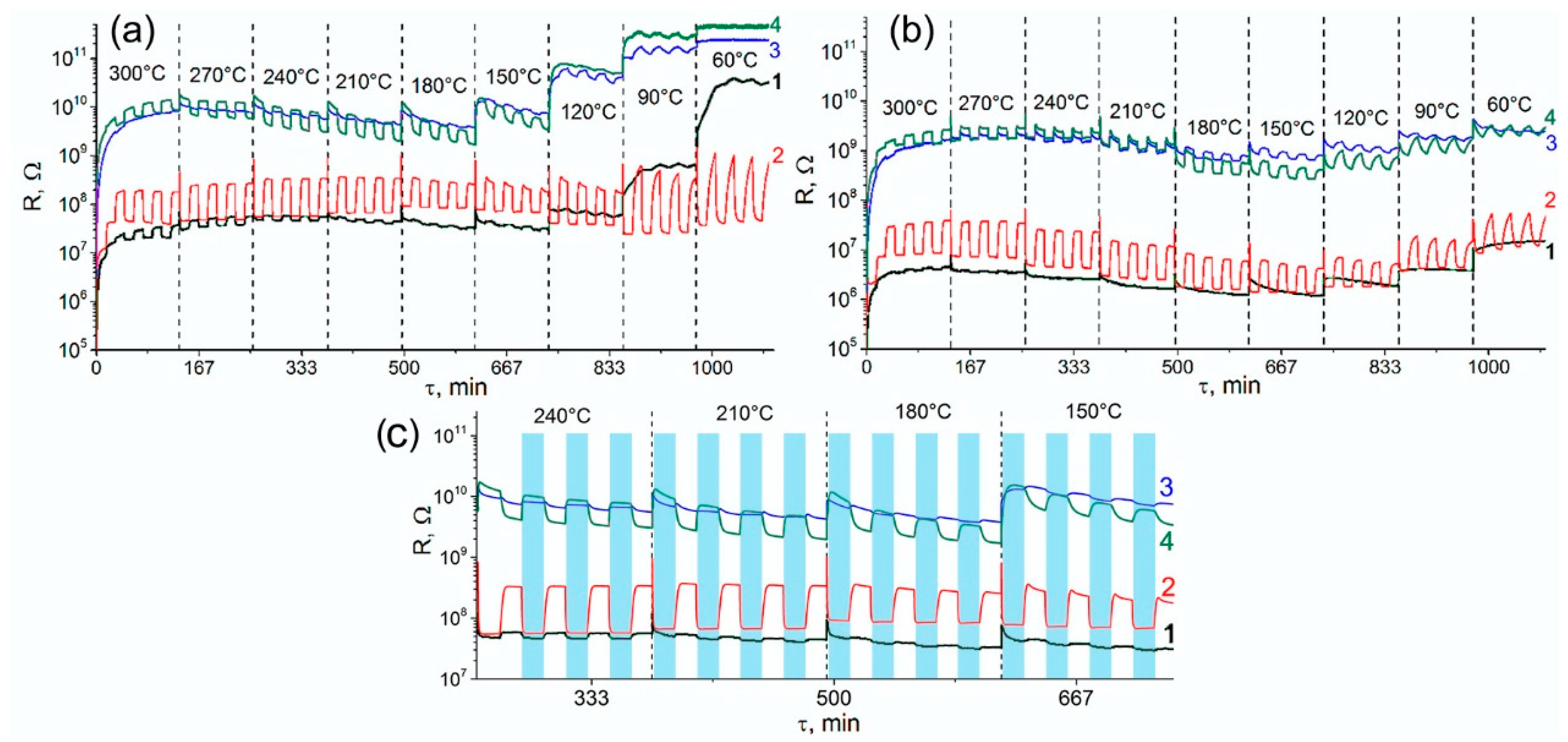
- Comparison of the resistance values show that the introduction of modifiers caused an increase in the resistance of tin dioxide. This effect was most pronounced for nanocomposites containing platinum.
- (ii)
- For the non-modified SnO2, the value of the n-type sensor response toward CO increased with increasing measurement temperature and reached a maximum at 270–300 °C. The increase in humidity almost completely suppressed the sensor response of SnO2 sample.
- (iii)
- For the SnO2/Pd nanocomposite, only the n-type response was observed. Two maxima can be distinguished on the temperature dependence of the sensor signal: one in the temperature range 240–270 °C and one in the range 60–90 °C. The sensor signal values at 240–270 °C decreased slightly with increasing air humidity from RH = 0% to RH = 20%. In the low-temperature interval, an increase in humidity led to a significant decrease in the sensor signal.
- (iv)
- The SnO2/Pt nanocomposite exhibited a low sensor response. However, in the low-temperature range, its response to CO in dry air exceeded the analogous value for unmodified SnO2, while in the high-temperature region its sensor signal turns out to be lower than for SnO2. The inversion of the sensor response from the n-type to the p-type is observed only when measured in dry air at T = 240 °C.
- (v)
- When performing the measurements in dry air, the inversion of the sensor response was characteristic for bimetallic nanocomposite SnO2/PtPd over a wide temperature range. The maximum of the p-type response was observed at 210–240 °C. The increase in air humidity led to the disappearance of the inversion of the response. The observed n-type response in the whole temperature range was lower than for palladium containing monometallic nanocomposite SnO2/Pd.
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structure and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Gurlo, A.; Bârsan, N.; Weimar, U. Gas Sensors Based on Semiconducting Metal Oxides. In Metal Oxides. Chemistry and Applications; Fierro, J.L.G., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 683–738. ISBN 978-0-8247-2371-2. [Google Scholar]
- Krivetskiy, V.V.; Rumyantseva, M.N.; Gaskov, A.M. Chemical modification of nanocrystalline tin dioxide for selective gas sensors. Russ. Chem. Rev. 2013, 82, 917–941. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Vorobyeva, N.A.; Rumyantseva, M.N.; Gaskov, A.M. Active sites on the surface of nanocrystalline semiconductor oxides ZnO and SnO2 and gas sensitivity. Russ. Chem. Bull. 2017, 66, 1728–1764. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- WHO. Air Quality Guidelines for Europe, 2nd ed.; WHO: Geneva, Switzerland, 2000; Available online: http://www.euro.who.int/__data/assets/pdf_file/0020/123059/AQG2ndEd_5_5carbonmonoxide.PDF?ua=1 (accessed on 29 August 2018).
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Savchenko, V.I. The Chemisorption of Oxygen and the Oxidation of Carbon Monoxide on Metals. Russ. Chem. Rev. 1986, 55, 222–231. [Google Scholar] [CrossRef]
- Over, H.; Muhler, M. Catalytic CO oxidation over ruthenium—Bridging the pressure gap. Prog. Surf. Sci. 2003, 72, 3–17. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kurokawa, Y.; Seiyama, T. Effects of additives on semiconductor gas sensors. Sens. Actuators B 1983, 4, 283–289. [Google Scholar] [CrossRef]
- Rella, R.; Siciliano, P.; Capone, S.; Epifani, M.; Vasanelli, L.; Licciulli, A. Air quality monitoring by means of sol–gel integrated tin oxide thin films. Sens. Actuators B 1999, 58, 283–288. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, A.; Morante, J.R.; Weimar, U.; Bârsan, N.; Göpel, W. Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO sol–gel nanocrystals for gas sensors. Sens. Actuators B 2000, 70, 87–100. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Boris, Y.; Ivanov, M.; Schwank, J.; Morante, J. Influence of surface Pd doping on gas sensing characteristics of SnO2 thin films deposited by spray pyrolysis. Thin Solid Films 2003, 436, 119–126. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Hwang, Y.K.; Jhung, S.H.; Mulla, I.S.; Chang, J.-S. Effect of Pt concentration on the physicochemical properties and CO sensing activity of mesostructured SnO2. Sens. Actuators B 2006, 114, 275–282. [Google Scholar] [CrossRef]
- Belmonte, J.C.; Manzano, J.; Arbiol, J.; Cirera, A.; Puigcorbe, J.; Vila, A.; Sabate, N.; Gracia, I.; Cane, C.; Morante, J.R. Micromachined twin gas sensor for CO and O2 quantification based on catalytically modified nano-SnO2. Sens. Actuators B 2006, 114, 881–892. [Google Scholar] [CrossRef]
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B 2006, 114, 283–295. [Google Scholar] [CrossRef]
- Koziej, D.; Barsan, N.; Shimanoe, K.; Yamazoe, N.; Szuber, J.; Weimar, U. Spectroscopic insights into CO sensing of undoped and palladium doped tin dioxide sensors derived from hydrothermally treated tin oxide sol. Sens. Actuators B 2006, 118, 98–104. [Google Scholar] [CrossRef]
- Dolbec, R.; El Khakania, M.A. Sub-ppm sensitivity towards carbon monoxide by means of pulsed laser deposited SnO2:Pt based sensors. Appl. Phys. Lett. 2007, 90, 173114. [Google Scholar] [CrossRef]
- Lee, Y.C.; Huang, H.; Tan, O.K.; Tse, M.S. Semiconductor gas sensor based on Pd-doped SnO2 nanorod thin films. Sens. Actuators B 2008, 132, 239–242. [Google Scholar] [CrossRef]
- Huang, H.; Ong, C.Y.; Guo, J.; White, T.; Tse, M.S.; Tan, O.K. Pt surface modification of SnO2 nanorod arrays for CO and H2 sensors. Nanoscale 2010, 2, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Marikutsa, A.V.; Rumyantseva, M.N.; Yashina, L.V.; Gaskov, A.M. Role of surface hydroxyl groups in promoting room temperature CO sensing by Pd-modified nanocrystalline SnO2. J. Solid State Chem. 2010, 183, 2389–2399. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Lian, G.; Yu, Q.; Luan, C.; Wang, Q.; Cui, D. Room temperature CO sensor fabricated from Pt-loaded SnO2 porous nanosolid. Sens. Actuators B 2013, 184, 33–39. [Google Scholar] [CrossRef]
- Zhukova, A.A.; Rumyantseva, M.N.; Zaytsev, V.B.; Zaytseva, A.V.; Abakumov, A.M.; Gaskov, A.M. Pd nanoparticles on SnO2(Sb) whiskers: Aggregation and reactivity in CO detection. J. Alloys Compd. 2013, 565, 6–10. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M. Specific interaction of PdOx- and RuOy-modified tin dioxide with CO and NH3 gases: Kelvin probe and drift studies. J. Phys. Chem. C 2015, 119, 24342–24350. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Sun, H.; Sun, P.; Wang, Y.; Lin, J.; Lu, G. Microwave assisted synthesis of hierarchical Pd/SnO2 nanostructuresfor CO gas sensor. Sens. Actuators B 2016, 222, 257–263. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Liu, F.; Liu, C.; Su, T.; Lin, J.; Sun, P.; Sun, Y.; Liu, F.; Lu, G. The enhanced CO gas sensing performance of Pd/SnO2 hollow sphere sensors under hydrothermal conditions. RSC Adv. 2016, 6, 80455–80461. [Google Scholar] [CrossRef]
- Guczi, L. Bimetallic nano-particles: Featuring structure and reactivity. Catal. Today 2005, 101, 53–64. [Google Scholar] [CrossRef]
- Rodriguez, J.A. Electronic and chemical properties of Pt, Pd and Ni in bimetallic surfaces. Surf. Sci. 1996, 345, 347–362. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Tian, P.; Zhou, J.; Tang, R.; Wu, S. A highly active and anti-coking Pd-Pt/SiO2 catalyst for catalytic combustion of toluene at low temperature. Appl. Catal. A 2017, 529, 60–67. [Google Scholar] [CrossRef]
- Calzada, L.A.; Collins, S.E.; Han, C.W.; Ortalan, V.; Zanella, R. Synergetic effect of bimetallic Au-Ru/TiO2 catalysts for complete oxidation of methanol. Appl. Catal. B 2017, 207, 79–92. [Google Scholar] [CrossRef]
- Toshima, N.; Kushihashi, K.; Yonezawa, T.; Hirai, H. Colloidal Dispersions of Palladium–Platinum Bimetallic Clusters Protected by Polymers. Preparation and Application to Catalysis. Chem. Lett. 1989, 18, 1769–1772. [Google Scholar] [CrossRef]
- Toshima, N.; Yonezawa, T.; Kushihashi, K. Polymer-protected palladium–platinum bimetallic clusters: Preparation, catalytic properties and structural considerations. J. Chem. Soc. Faraday Trans. 1993, 89, 2537–2543. [Google Scholar] [CrossRef]
- Cho, S.J.; Kang, S.K. Structural transformation of PdPt nanoparticles probed with X-ray absorption near edge structure. Catal. Today 2004, 93, 561–566. [Google Scholar] [CrossRef]
- Endo, T.; Kuno, T.; Yoshimura, T.; Esumi, K. Preparation and Catalytic Activity of Au–Pd, Au–Pt, and Pt–Pd Binary Metal Dendrimer Nanocomposites. J. Nanosci. Nanotechnol. 2005, 5, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhao, M.; Vora, M.; Shen, J.; Zeng, C.; Yan, W.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Synergistic Effects of Bimetallic PtPd/TiO2 Nanocatalysts in Oxidation of Glucose to Glucaric Acid: Structure Dependent Activity and Selectivity. Ind. Eng. Chem. Res. 2016, 55, 2932–2945. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, L.; He, Y.; Wang, L.; Huang, Z.; Jiang, Y.; Gao, J. Mesoporous Bimetallic PtPd Nanoflowers as a Platform to Enhance Electrocatalytic Activity of Acetylcholinesterase for Organophosphate Pesticide Detection. Elecroanalysis 2018, 30, 1801–1810. [Google Scholar] [CrossRef]
- Zhang, J.; Gan, W.; Fu, X.; Hao, H. A microwave assisted one-pot route synthesis of bimetallic PtPd alloy cubic nanocomposites and their catalytic reduction for 4-nitrophenol. Mater. Res. Express 2017, 4, 105022. [Google Scholar] [CrossRef] [Green Version]
- Navarro, R.M.; Pawelec, V.; Trejo, J.M.; Mariscal, R.; Fierro, J.L.G. Hydrogenation of Aromatics on Sulfur-Resistant PtPd Bimetallic Catalysts. J. Catal. 2000, 189, 184–194. [Google Scholar] [CrossRef]
- Xia, T.; Shen, H.; Chang, G.; Zhang, Y.; Shu, H.; Oyama, M.; He, Y. Facile and Rapid Synthesis of Ultrafine PtPd Bimetallic Nanoparticles and Their High Performance toward Methanol Electrooxidation. J. Nanomater. 2014, 2014, 496249. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials. Properties, Advantages and Shortcomungs for Applications. Vol. 1. Convenional Approaches; Springer Science +Business Media, LLC: New York, NY, USA, 2013; pp. 279–281. ISBN 978-1-4614-7164-6. [Google Scholar]
- Zhang, T.; Liu, L.; Qi, Q.; Li, S.; Lu, G. Development of microstructure In/Pd-doped SnO2 sensor for low-level CO detection. Sens. Actuators B 2009, 139, 287–291. [Google Scholar] [CrossRef]
- Yin, X.-T.; Guo, X.-M. Selectivity and sensitivity of Pd-loaded and Fe-doped SnO2sensor for CO detection. Sens. Actuators B 2014, 200, 213–218. [Google Scholar] [CrossRef]
- Mutinati, G.C.; Brunet, E.; Koeck, A.; Steinhauer, S.; Yurchenko, O.; Laubender, E.; Urban, G.; Siegert, J.; Rohracher, K.; Schrank, F.; et al. Optimization of CMOS integrated nanocrystalline SnO2 gas sensor devices with bimetallic nanoparticles. Procedia Eng. 2014, 87, 787–790. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Cho, H.-J.; Koo, W.-T.; Tuller, H.L.; Kim, I.-D. Exceptional High-Performance of Pt-Based Bimetallic Catalysts for Exclusive Detection of Exhaled Biomarkers. Adv. Mater. 2017, 29, 1700737. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.A.; Mulla, I.S.; Vijayamohanan, K. Synergetic sensitivity effects in surface modified tin oxide hydrogen sensors using ruthenium and palladium oxides. J. Mater. Sci. Lett. 1997, 16, 1819–1821. [Google Scholar] [CrossRef]
- Chaudhary, V.A.; Mulla, I.S.; Vijayamohanan, K. Selective hydrogen sensing properties of surface functionalized tin oxide. Sens. Actuators B 1999, 55, 154–160. [Google Scholar] [CrossRef]
- Choi, U.-S.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Sensing properties of Au-loaded SnO2–Co3O4 composites to CO and H2. Sens. Actuators B 2005, 107, 397–401. [Google Scholar] [CrossRef]
- Vladimirova, S.A.; Rumyantseva, M.N.; Filatova, D.G.; Kozlovskii, V.F.; Chizhov, A.S.; Khmelevskii, N.O.; Marchevskii, A.V.; Li, X.; Gaskov, V. SnO2(Au0, CoII,III) Nanocomposites: A Synergistic Effect of the Modifiers in CO Detection. Inorg. Mater. 2016, 52, 94–100. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Rumyantseva, M.N.; Nikolaev, S.A.; Marikutsa, A.V.; Smirnov, V.V.; Alikhanian, A.S.; Gaskov, A.M. Effect of Au and NiO catalysts on the NO2 sensing properties of nanocrystalline SnO2. Inorg. Mater. 2010, 46, 232–236. [Google Scholar] [CrossRef]
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, S.S. Bimetallic Pd/Pt nanoparticle-functionalized SnO2 nanowires for fast response and recovery to NO2. Sens. Actuators B 2013, 181, 446–453. [Google Scholar] [CrossRef]
- Malkov, I.V.; Krivetskiy, V.V.; Potemkin, D.I.; Zadesenets, A.V.; Batuk, M.M.; Hadermann, J.; Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M. Effect of Bimetallic Pd/Pt Clusters on the Sensing Properties of Nanocrystalline SnO2 in the Detection of CO. Russ. J. Inorg. Chem. 2018, 63, 1007–1011. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Ponzoni, A.; Comini, E.; Badalyan, S.; Rumyantseva, M.; Gaskov, A. Selectivity Modification of SnO2-Based Materials for Gas Sensor Arrays. Electroanalysis 2010, 22, 2809–2816. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amala, R.; Mâdler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, J.A.; Pokhrel, S.; Mädler, L.; Weimar, U.; Bârsan, N. Flame spray pyrolysis for sensing at the nanoscale. Nanotechnology 2013, 24, 442001. [Google Scholar] [CrossRef] [PubMed]
- Koirala, R.; Pratsinisa, S.E.; Baiker, A. Synthesis of catalytic materials in flames: Opportunities and challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef] [PubMed]
- Inyawilert, K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Ultra-sensitive and highly selective H2 sensors based on FSP-made Rh-substituted SnO2 sensing films. Sens. Actuators B 2017, 240, 1141–1152. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Jiang, H.; Hou, X.; Li, C. Construction of core–shell Fe2O3@SnO2 nanohybrids for gas sensors by a simple flame-assisted spray process. RSC Adv. 2013, 3, 22373–22379. [Google Scholar] [CrossRef]
- Tricoli, A.; Graf, M.; Pratsinis, S.E. Optimal Doping for Enhanced SnO2 Sensitivity and Thermal Stability. Adv. Funct. Mater. 2008, 18, 1969–1976. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Phanichphant, S. H2 Sensing Response of Flame-Spray-Made Ru/SnO2 Thick Films Fabricated from Spin-Coated Nanoparticles. Sensors 2009, 9, 8996–9010. [Google Scholar] [CrossRef] [PubMed]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Phanichphant, S. The effect of Pt nanoparticles loading on H2 sensing properties of flame-spray-made SnO2 sensing films. Mater. Chem. Phys. 2014, 147, 661–672. [Google Scholar] [CrossRef]
- Sukunta, J.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Highly-sensitive H2S sensors based on flame-made V-substituted SnO2 sensing films. Sens. Actuators B 2017, 242, 1095–1107. [Google Scholar] [CrossRef]
- Kotchasak, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Yordsri, V.; Liewhiran, C. Highly sensitive and selective detection of ethanol vapor using flame-spray-made CeOx-doped SnO2 nanoparticulate thick films. Sens. Actuators B 2018, 255, 8–21. [Google Scholar] [CrossRef]
- Rebholz, J.; Jaeschke, C.; Hübner, M.; Pham, D.; Mädler, L.; Weimar, U.; Bârsan, N. Conduction Mechanism in Undoped and Antimony Doped SnO2 Based FSP Gas Sensors. In Proceedings of the 14th International Meeting on Chemical Sensors, Nuremberg, Germany, 20–23 May 2012; pp. 105–108. [Google Scholar] [CrossRef]
- XPS Interpretation of Palladium. Available online: https://xpssimplified.com/elements/palladium.php (accessed on 14 October 2018).
- XPS Interpretation of Platinum. Available online: https://xpssimplified.com/elements/platinum.php (accessed on 14 October 2018).
- Liu, Y.; Yang, F.; Yang, X. Size-controlled synthesis and characterization of quantum-size SnO2 nanocrystallites by a solvothermal route. Colloids Surf. A 2008, 312, 219–225. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Konstantinova, E.A.; Shatalova, T.B.; Gaskov, A.M. Active sites on nanocrystalline tin dioxide surface: Effect of palladium and ruthenium oxides clusters. J. Phys. Chem. C 2014, 118, 21541–21549. [Google Scholar] [CrossRef]
- Conner, W.C.; Falconer, J.L. Spillover in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Frolov, D.D.; Morozov, I.V.; Boltalin, A.I.; Fedorova, A.A.; Petukhov, I.A.; Yashina, L.V.; Konstantinova, E.A.; Sadovskaya, V.; et al. Role of PdOx and RuOy clusters in oxygen exchange between nanocrystalline tin dioxide and the gas phase. J. Phys. Chem. C 2013, 117, 23858–23867. [Google Scholar] [CrossRef]
- Descorme, C.; Duprez, D. Oxygen surface mobility and isotopic exchange on oxides: Role of the nature and the structure of metal particles. Appl. Catal. A 2000, 202, 231–241. [Google Scholar] [CrossRef]
- Morrison, S.R. The Chemical Physics of Surfaces, 2nd ed.; Springer Science +Business Media, LLC: New York, NY, USA, 1990; pp. 29–31. ISBN 978-1-4899-2500-8. [Google Scholar]
- Jaeckel, R.; Wagner, B. Photo-electric measurement of the work function of metals and its alteration after gas adsorption. Vacuum 1963, 13, 509–511. [Google Scholar] [CrossRef]
- Ganose, A.M.; Scanlon, D.O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaic. J. Mater. Chem. C 2016, 4, 1467–1475. [Google Scholar] [CrossRef]
- Lalik, E.; Kosydar, R.; Tokarz-Sobieraj, R.; Witko, M.; Szumełda, T.; Kołodziej, M.; Rojek, W.; Machej, T.; Bielańska, E.; Drelinkiewicz, A. Humidity induced deactivation of Al2O3 and SiO2 supported Pd, Pt, Pd-Pt catalysts in H2 + O2 recombination reaction: The catalytic, microcalorimetric and DFT studies. Appl. Catal. A 2015, 501, 27–40. [Google Scholar] [CrossRef]
- Degler, D.; Pereira de Carvalho, H.W.; Kvashnina, K.; Grunwaldt, J.-D.; Weimar, U.; Bârsan, N. Structure and chemistry of surface-doped Pt:SnO2 gas sensing materials. RSC Adv. 2016, 6, 28149–28155. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]
- Benesh, G.A.; Liyanage, L.S.G. The surface electronic structure of oxygen on Pt(001)(1×1). Surf. Sci. 1992, 261, 207–216. [Google Scholar] [CrossRef]
- Sales, B.C.; Turner, J.E.; Maple, B.M. Oscillatory oxidation of CO over Pt, Pd and Ir catalysts: Theory. Surf. Sci. 1982, 114, 381–394. [Google Scholar] [CrossRef]
- Bârsan, N.; Rebholz, J.; Weimar, U. Conduction mechanism switch for SnO2 based sensors during operation in application relevant conditions; implications for modeling of sensing. Sens. Actuators B 2015, 207, 455–459. [Google Scholar] [CrossRef]
- Prasad, A.K.; Kubinski, D.J.; Gouma, P.I. Comparison of sol–gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection . Sens. Actuators B 2003, 93, 25–30. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Golovanov, V.; Cerneavschi, A.; Matolin, V.; Todd, A. Acceptor-like behavior of reducing gases on the surface of n-type In2O3. Appl. Surf. Sci. 2004, 227, 122–131. [Google Scholar] [CrossRef]
- Galatsis, K.; Cukrov, L.; Wlodarski, W.; McCormick, P.; Kalantar-zadeh, K.; Comini, E.; Sberveglieri, G. p- and n-type Fe-doped SnO2 gas sensors fabricated by the mechanochemical processing technique. Sens. Actuators B 2003, 93, 562–565. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Konstantinova, E.; Grishina, D.; Gaskov, A. Inversion of NH3 sensor signal and paramagnetic centers of nanocrystalline ZnO(Ga). Procedia Eng. 2011, 25, 296–299. [Google Scholar] [CrossRef]
- Shekunova, T.O.; Baranchikov, A.E.; Yapryntsev, A.D.; Rudakovskaya, P.G.; Ivanova, O.S.; Karavanova, Y.A.; Kalinina, M.A.; Rumyantseva, M.N.; Dorofeev, S.G.; Ivanov, V.K. Ultrasonic disintegration of tungsten trioxide pseudomorphs after ammonium paratungstate as a route for stable aqueous sols of nanocrystalline WO3. J. Mater. Sci. 2018, 53, 1758–1768. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Hu, M.; Wei, X.-Y. A study of transition from n- to p-type based on hexagonal WO3 nanorods sensor. Chin. Phys. B 2014, 23, 40704. [Google Scholar] [CrossRef]
- Kim, I.-D.; Rothschild, A.; Lee, B.H.; Kim, D.Y.; Jo, S.M.; Tuller, H.L. Ultrasensitive Chemiresistors Based on Electrospun TiO2 Nanofibers. Nano Lett. 2006, 6, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Gurlo, A.; Sahm, M.; Oprea, A.; Barsan, N.; Weimar, U. A p- to n-transition on α-Fe2O3-based thick film sensors studied by conductance and work function change measurements. Sens. Actuators B 2004, 102, 291–298. [Google Scholar] [CrossRef]

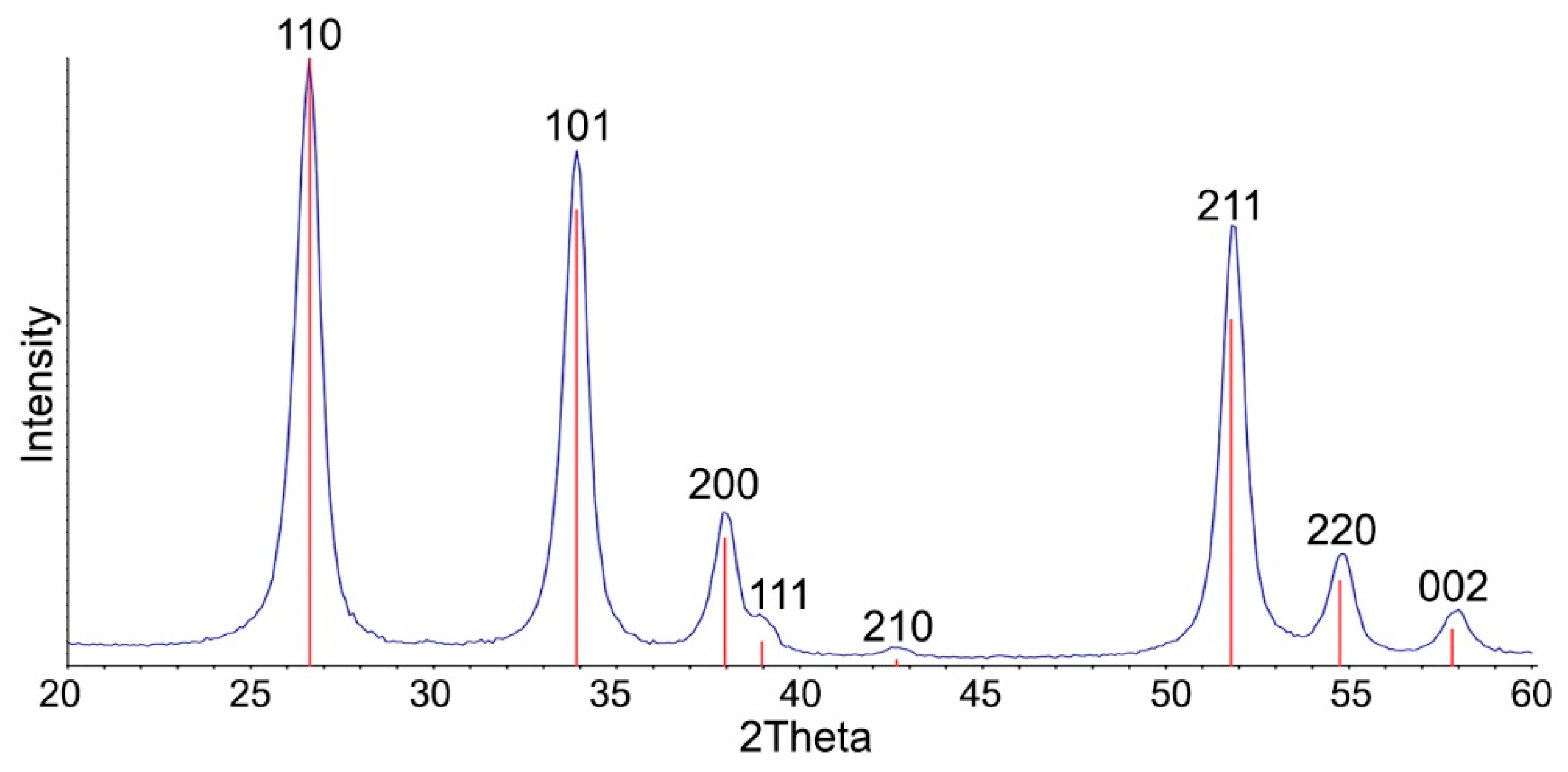



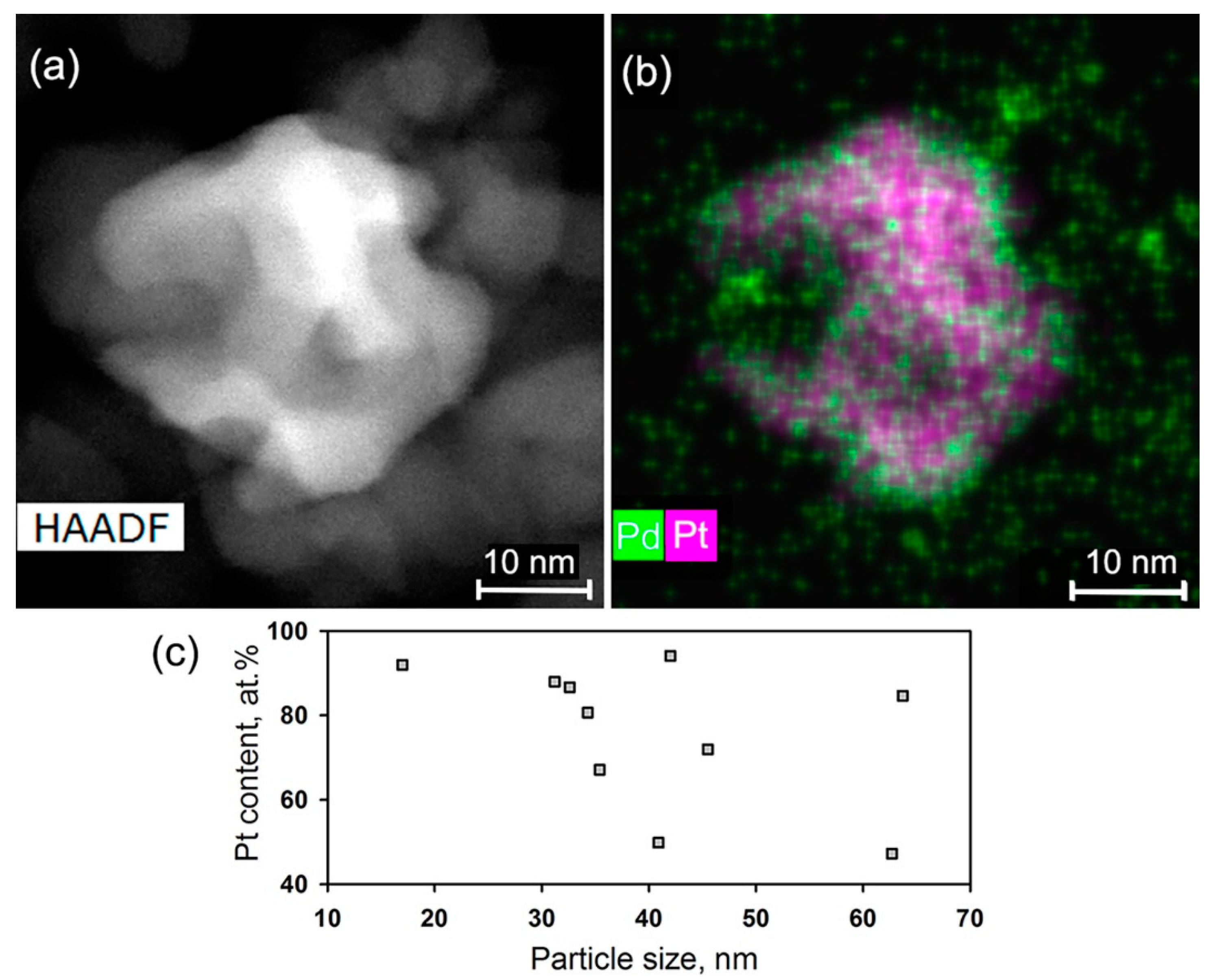


| Sample | Ssurf, m2/g | dXRD (SnO2), nm (a) | dTEM, nm (b) | [M], wt.% (c) | ||
|---|---|---|---|---|---|---|
| SnO2 | Pd | Pt | ||||
| SnO2 | 22 ± 5 | 10 ± 1 | 10.7 ± 4.9 | - | - | - |
| SnO2/Pd | <2; 8–20 | - | 1.5 ± 0.2 (c) | |||
| SnO2/Pt | - | 25–100 | 1.0 ± 0.2 (c) | |||
| SnO2/PtPd | <2 | 17–64 | 1.3 ± 0.2 (Pd) 0.3 ± 0.1 (Pt) | |||
| Spectral Assignment | Binding Energy, eV | ||||
|---|---|---|---|---|---|
| Ref. [65] | Ref. [66] | SnO2/Pd | SnO2/Pt | SnO2/PtPd | |
| Pd 3d5/2 | Pd (0) 335.4 | 336.9 | - | 336.0 | |
| PdO 336.4 | |||||
| Pt 4f7/2 | Pt (0) 71.0 | - | 72.0 | 72.2 | |
| Pt (II) 72.4 | |||||
| Pt (IV) 74.9 | |||||
| Sample | Hydrogen Consumption, Mol H2 per 1 Mol SnO2 | Tmax, °C | |||
|---|---|---|---|---|---|
| Total | 100–300 °C | 370–850 °C | |||
| SnO2 | 2.9 ± 0.3 | 0.7 ± 0.1 | 2.2 ± 0.5 | 630 | 1.0×10−4 |
| SnO2/Pd | 2.5 ± 0.3 | 0.2 ± 0.1 | 2.3 ± 0.5 | 580 | 3.0×10−5 |
| SnO2/Pt | 2.3 ± 0.3 | 0.4 ± 0.1 | 1.9 ± 0.5 | 565 | 6.0×10−5 |
| SnO2/PtPd | 2.1 ± 0.3 | 0.2 ± 0.1 | 1.9 ± 0.5 | 565 | 3.0×10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutukov, P.; Rumyantseva, M.; Krivetskiy, V.; Filatova, D.; Batuk, M.; Hadermann, J.; Khmelevsky, N.; Aksenenko, A.; Gaskov, A. Influence of Mono- and Bimetallic PtOx, PdOx, PtPdOx Clusters on CO Sensing by SnO2 Based Gas Sensors. Nanomaterials 2018, 8, 917. https://doi.org/10.3390/nano8110917
Kutukov P, Rumyantseva M, Krivetskiy V, Filatova D, Batuk M, Hadermann J, Khmelevsky N, Aksenenko A, Gaskov A. Influence of Mono- and Bimetallic PtOx, PdOx, PtPdOx Clusters on CO Sensing by SnO2 Based Gas Sensors. Nanomaterials. 2018; 8(11):917. https://doi.org/10.3390/nano8110917
Chicago/Turabian StyleKutukov, Pavel, Marina Rumyantseva, Valeriy Krivetskiy, Darya Filatova, Maria Batuk, Joke Hadermann, Nikolay Khmelevsky, Anatoly Aksenenko, and Alexander Gaskov. 2018. "Influence of Mono- and Bimetallic PtOx, PdOx, PtPdOx Clusters on CO Sensing by SnO2 Based Gas Sensors" Nanomaterials 8, no. 11: 917. https://doi.org/10.3390/nano8110917
APA StyleKutukov, P., Rumyantseva, M., Krivetskiy, V., Filatova, D., Batuk, M., Hadermann, J., Khmelevsky, N., Aksenenko, A., & Gaskov, A. (2018). Influence of Mono- and Bimetallic PtOx, PdOx, PtPdOx Clusters on CO Sensing by SnO2 Based Gas Sensors. Nanomaterials, 8(11), 917. https://doi.org/10.3390/nano8110917








