Abstract
A chitosan-MgO hybrid nanocomposite was prepared using a simple chemical precipitation method and characterized using Fourier transform spectroscopy (FTIR), elemental analysis (EDX), and scanning electron microscopy (SEM). The nanocomposite was served as a powerful ecofriendly basic catalyst under microwave irradiation in the synthesis of two novel series of 5-arylazo-2-hydrazonothiazoles 4a–j and 2-hydrazono[1,3,4]thiadiazoles 8a–d, incorporating a sulfonamide group. The structures of the synthesized products were elucidated by spectral data and elemental analyses. Also, their yield percentages were calculated using triethylamine (as a traditional catalyst) and chitosan-MgO nanocomposite (as a green recyclable catalyst) in a comparative study.
1. Introduction
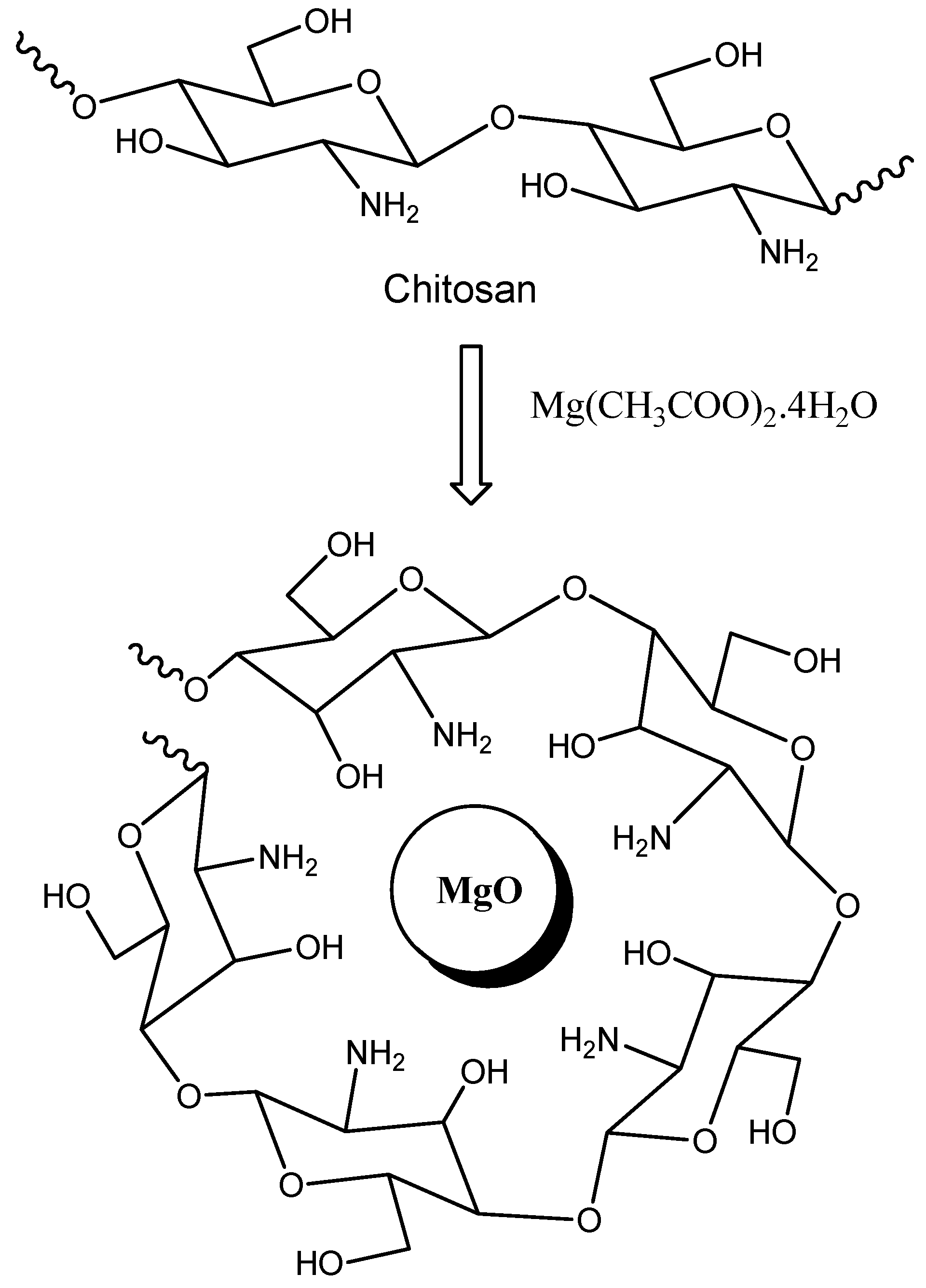
In the past decade, green chemistry has been developed from a variety of research ideas such as atom economy and heterogeneous catalysis in the last few decades leading up to the 1990s, and in several successful attempts and efforts to overcome the problems of chemical pollution and resource depletion. On the other hand, nanosized materials are of great importance due to their large exposed surface area, high absorbability, and high catalytic efficiency. Nanosized magnesium oxides were being multitalented basic catalysts for many organic reactions. Recently, MgO nanoparticles can be used for the synthesis of pyranopyrazoles [1], aminochromenes [2], and dihydropyridines [3] via multicomponent reactions. The main drawbacks to the potential use of MgO nanoparticles as a basic catalyst are their difficult separation and reusability since the utilized catalyst could not recover quantitatively and the pure products should be obtained after extensive purification processes. Chitosan (CS) is a natural polysaccharide that is commercially produced via the alkaline hydrolysis of chitin. Although this biopolymer has many advantages, such as its renewability, biocompatibility, and biodegradability, its utility is limited in its unmodified form. For a long time, chitosan was used as an ecofriendly basic catalyst in some organic reactions [4,5,6,7]. However, the major problem of its use is the limited basic properties (weak catalytic activity) and its high swelling properties and gel formation renders its separation and recovery very difficult. To overcome these drawbacks, a chitosan-MgO nanocomposite [8] could be used as a novel basic heterogeneous catalyst in the form of a solid film. A chitosan-MgO nanocomposite is a three-dimensional, cross-linked, polymeric matrix of chitosan (with active NH and OH functional groups) incorporating magnesium oxide nanoparticles (Figure 1) [9].
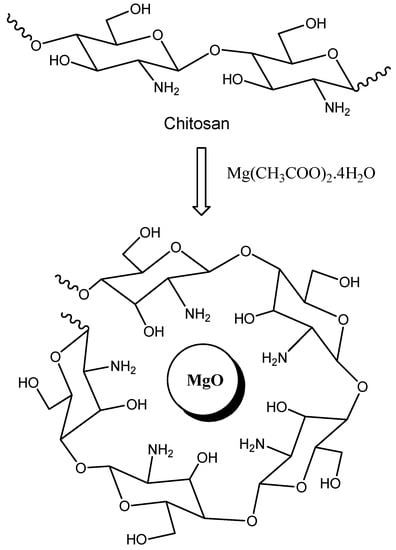
Figure 1.
Structure of a chitosan-MgO nanocomposite.
The biocatalyst is stable enough such that it can be recovered and used more than four times without a loss in its catalytic activity. As a result of these studies, the chitosan-MgO biocatalyst was found to be ecofriendly, biocompatible, an efficient green catalyst, and a high economic impact material that will find a lot of potential catalytic applications, especially in the base catalyzed organic transformations. This heterogeneous hybrid nanocomposite is used in the form of films to increase its catalytic activity by increasing its surface/volume ratio, and consequently it can be easily recycled and reused several times with the same catalytic efficiency [10].
Moreover, thiazoles display a diversity of therapeutic activities such as being a non-steroidal anti-inflammatory drug (meloxicam), antiretroviral drug (ritonavir), antiprotozoal agent (nitazoxanide) [11], and antioxidant agent [12]. Also, [1,3,4]thiadiazoles with a sulfonamide group were incorporated in many marketing drugs including acetazolamide and methazolamide [13]. In this study, we are reporting an efficient green protocol and simple synthetic routes of a new series of hydrazonothiazoles and hydrazono [1,3,4]thiadiazoles in the presence of chitosan-MgO nanocomposite as a basic ecofriendly biocatalyst under microwave irradiations. Moreover, the efficiency of this biocatalyst and its reusability was examined.
2. Materials and Methods
Melting points were measured on an electrothermal Gallenkamp capillary apparatus (Leicester, UK) and are uncorrected. Elemental analyses were carried out at the Microanalytical Center of Cairo University, Giza, Egypt. The IR spectra were recorded on a Pye-Unicam SP300 Instrument (Cambridge, UK) in potassium bromide discs. The 1H NMR (nuclear magnetic resonance) and 13C NMR of the newly synthesized compounds in DMSO-d6 were measured on a Varian Mercury VXR-300 spectrometer (Varian, Karlsruhe, Germany) at 300 MHz for 1H NMR and 75 MHz for 13C NMR) and the chemical shifts were related to that of the solvent. The mass spectra were recorded on a GCMSQ1000-EX Shimadzu (Tokyo, Japan) and GCMS 5988-A HP spectrometers (Shimadzu, Tokyo, Japan) where the ionizing voltage was 70 eV. Microwave experiments were carried out using CEM Discover Labmate microwave apparatus (Discover, SP, NC, USA, 300 W). The starting materials 2-{1-[4-((4-methylphenyl)sulfonamide) phenyl]ethylidine}thiosemicarbazide 1 [14], α-keto hydrazonoyl halides 2a–j [15,16,17,18], and arenecarbohydrazonoyl halides 5a–d [19,20] were prepared as reported in literature.
2.1. Preparation of Heterogeneous Catalyst (Chitosan-MgO Hybrid Nanocomposite)
The hybrid CS-MgO nanocomposite was prepared using a one pot solution casting method. In an experiment, 2 g of chitosan powder (medium molecular weight; 85% DDA) was dissolved in 100 mL of 2% (v/v) aqueous acetic acid solution under stirring for 12 h at room temperature. To this solution, 2 g of magnesium acetate tetrahydrate, Mg(CH3COO)2·4H2O (M0631 Sigma-Aldrich, St. Louis, MO, USA) were added and the mixture was again re-stirred for 12 h till a clear solution was obtained. The resulting viscous solution was cast in a Teflon Petri dish and dried overnight at room temperature. The Petri dish was then immersed into a 0.2 M sodium hydroxide solution for neutralization and phase separation. The solid films were dried in an oven adjusted at 80 °C for 6 h. Finally, the produced solid films were purified by washing with methanol several times and again were dried in an oven at 60 °C for 2 h. Furthermore, a pure chitosan film, without MgO, was prepared in a similar way for comparative study.
2.2. Reactions of 2-{1-[4-((4-Methylphenyl)sulfonamide)phenyl]ethylidine}thiosemicarbazide (1) with α-Keto Hydrazonoyl Chlorides 2a–j or N-Aryl Arenecarbohydrazonoyl Halides 5a–d
2.2.1. Method A
A mixture of 2-{1-[4-((4-methylphenyl)sulfonamide)phenyl]ethylidine}thiosemicarbazide (1) (0.362 g, 1 mmol) in dry dioxane (15 mL), containing 0.1 g of triethylamine, and α-keto hydrazonoyl chlorides 2a–j or N-aryl arenecarbohydrazonoyl halides 5a–d (1 mmol of each) was irradiated using microwave irradiation (MW) at 300 W in a closed Teflon vessel until all the starting material was consumed (30–40 min as monitored by thin layer chromatography, TLC). The solvent was evaporated and the residue was poured into an ice/HCl mixture. The precipitate was filtered, washed with water, and crystallized from methanol to give products 4a–j or 8a–d.
2.2.2. Method B
The procedure was similar to Method A, using a chitosan-MgO nanocomposite (0.1 g) instead of trimethylamine. After completion of the reaction, the hot solution was filtered to remove chitosan-MgO nanocomposite and the filtrate was poured into an ice/HCl mixture, and the precipitate was filtered, washed with water, and crystallized from methanol to give products 4a–j or 8a–d (see Supplementary Materials for analyses of the prepared compounds).
3. Results and Discussion
3.1. Preparation and Characterization of Chitosan-MgO Nanocomposite Films
The chitosan-MgO nanocatalyst was prepared via incorporation of the MgO nanoparticles in the chitosan matrix through a modified one pot solution casting method. The chitosan solution in acetic acid was treated with magnesium acetate tetrahydrate, then was subjected to evaporation of the solvent at room temperature. The resulting solid film was soaked in sodium hydroxide solution to achieve the phase separation.
3.1.1. FTIR Spectra
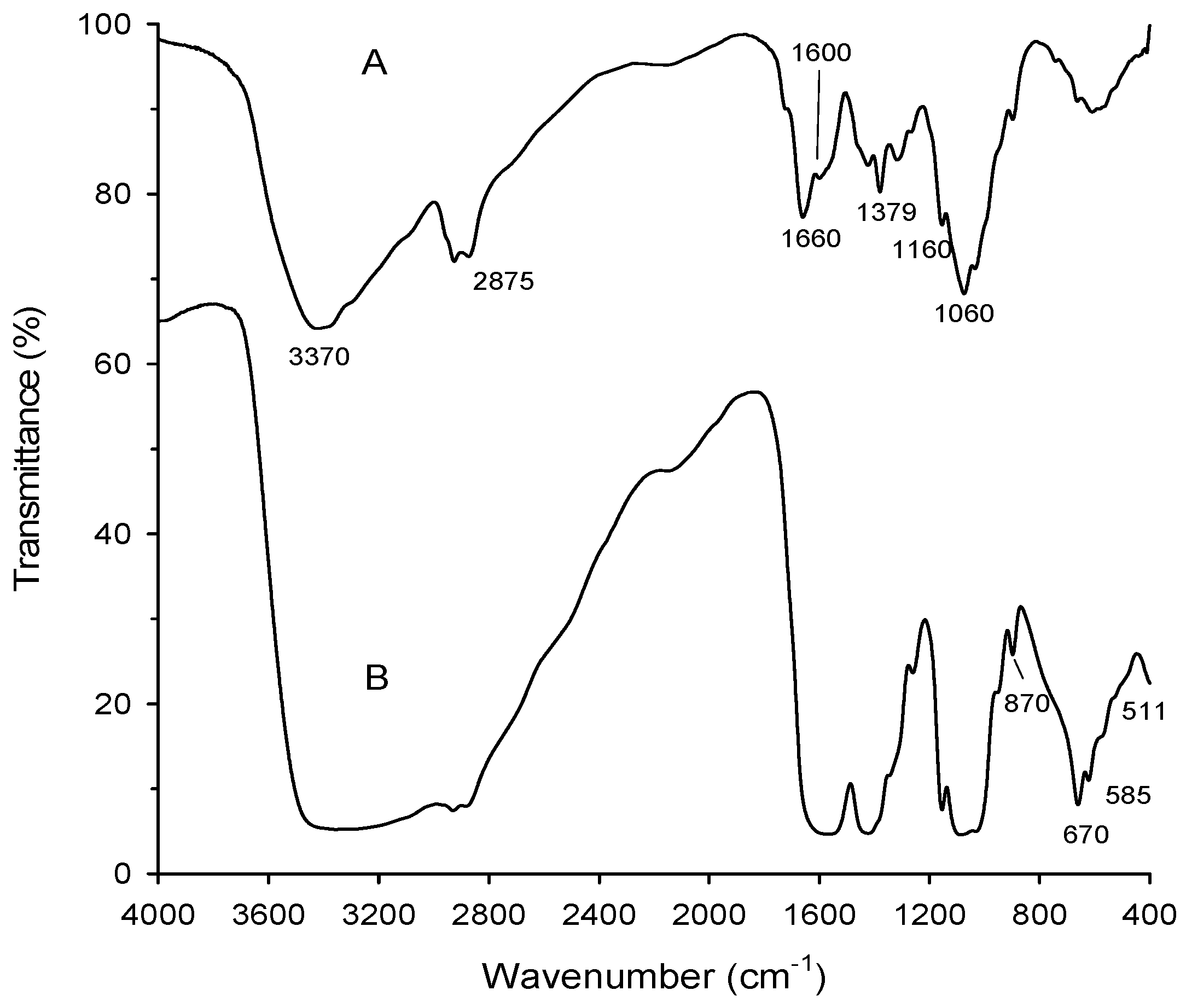
FTIR (Fourier-Transform infrared) spectra of chitosan and chitosan-MgO nanocomposite showed that the main functional groups of chitosan clearly appeared at υ = 3370 cm−1 (broad band of hydrogen bonded OH– groups), 2875 cm−1 (C–H bond; CH3 groups), 1660 cm−1 (amide carbonyl groups), 1379 cm−1 (bending vibration of CH2 groups), and 1060 cm−1 (asym. vibration of C=O) (Figure 2). These bands are considered as evidence for the maintenance of the chitosan structure features even after the incorporation of the MgO nanoparticles inside the polymer matrix. Also, the absence of acetic acid bands in the spectrum indicated that the films were washed enough and neutralized completely upon sodium hydroxide treatment (see Section 2.1). Moreover, only small acceptable shift in the bands of chitosan-MgO nanocomposite was attributed to the influence of the incorporation of MgO nanoparticles. This shift in bands was familiar as result of chitosan with metal oxides [21]. The latter shifts are shown, especially at bands of NH and OH groups, which is evidence for the H-bonding interaction of these group with MgO molecules.
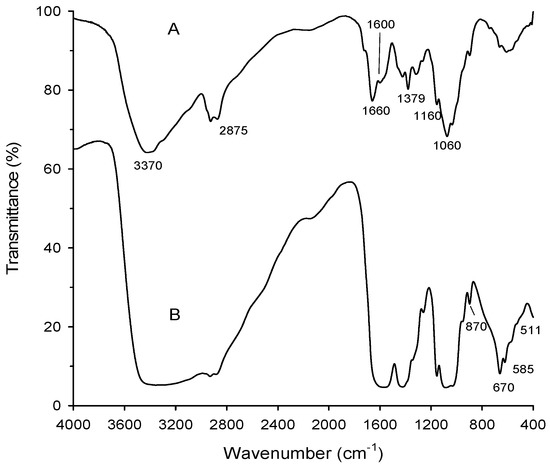
Figure 2.
FTIR of chitosan (A) and the chitosan-MgO nanocomposite (B).
3.1.2. FESEM Analysis
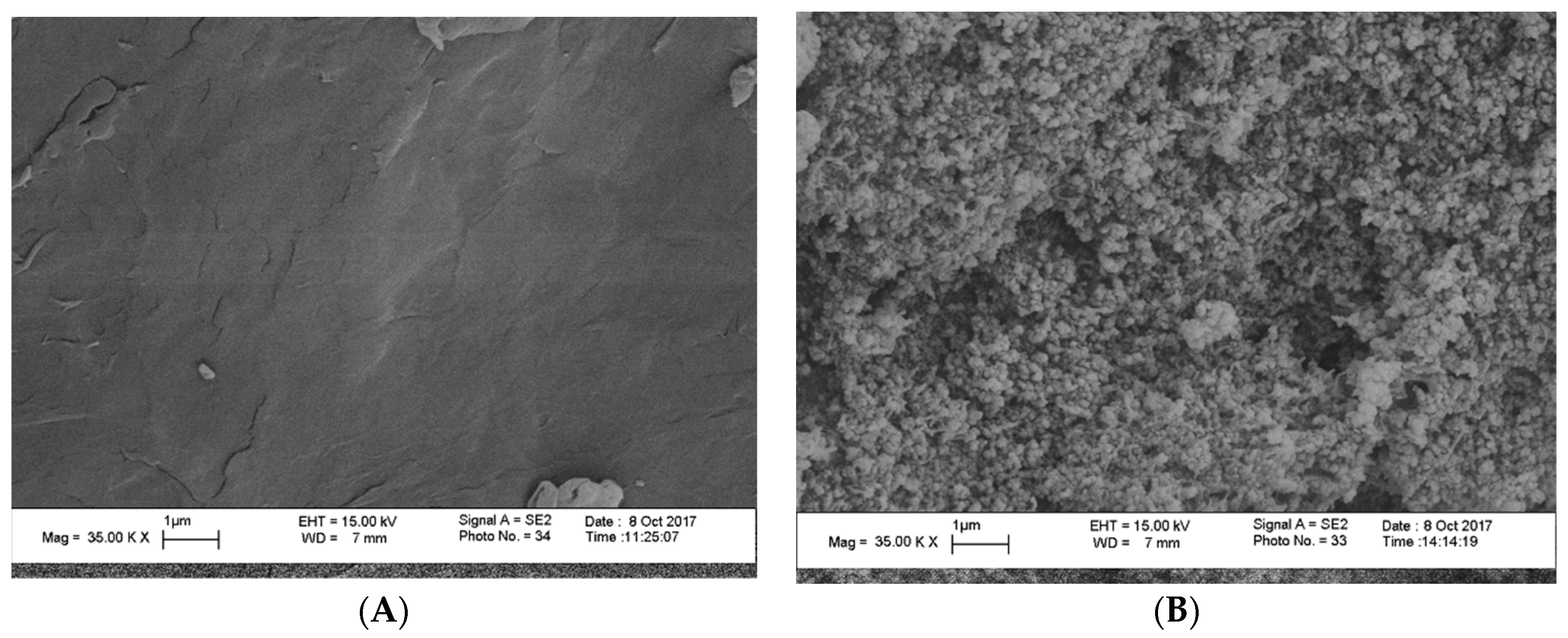
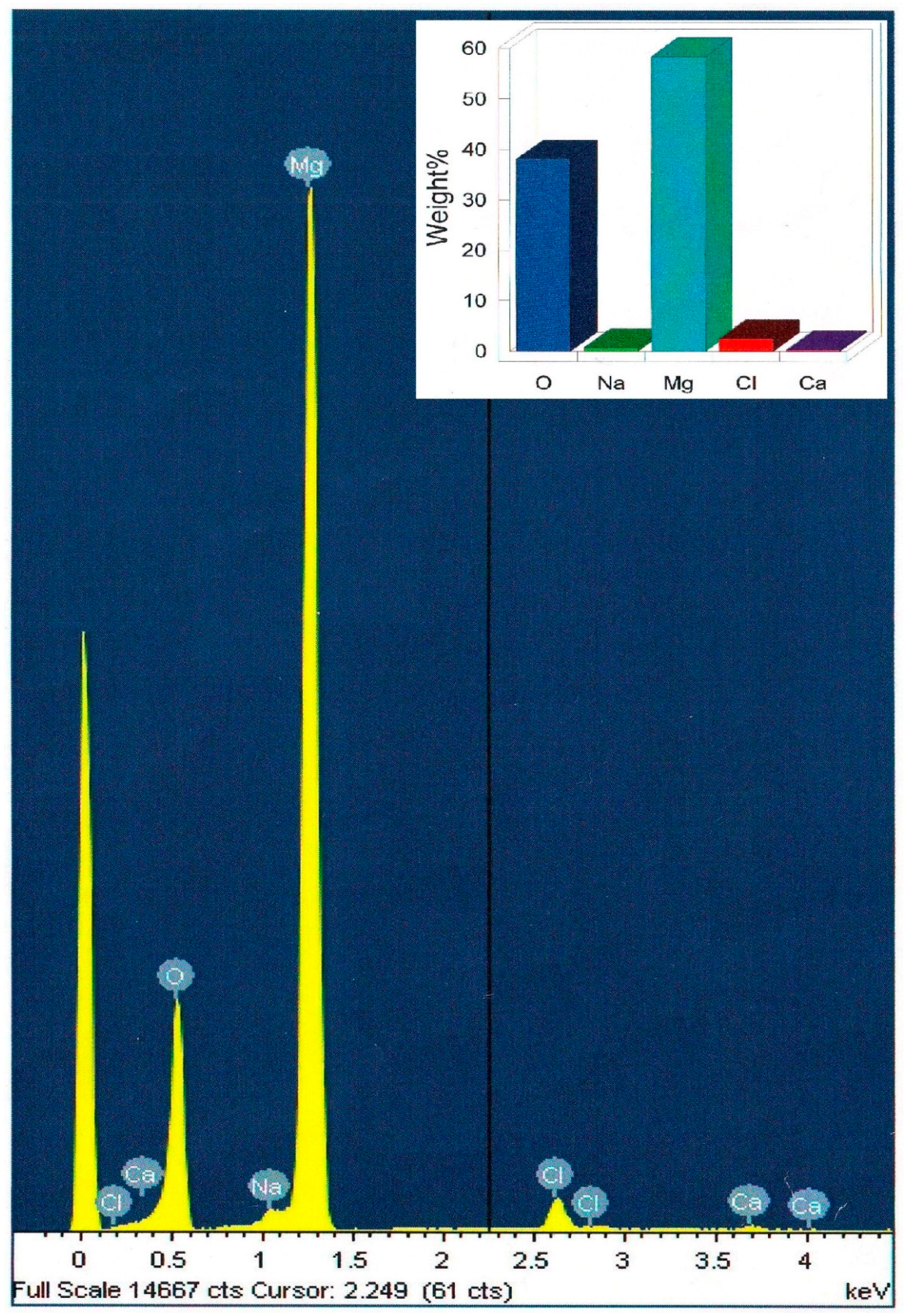
FESEM (Field Emission Scanning Electron Microscope) was used to analyze the morphology and size distribution of the MgO nanoparticles that were incorporated in the polymer matrix. FESEM micrographs of the pure chitosan (A) and that of the hybrid films with magnesia particles 10 wt% (B) are shown in (Figure 3). The obtained surface of the pure chitosan matrix was found to be homogenous and looks smoothly to a great extent. The MgO nanoparticles developed as white spots that distributed homogeneously over the surface of the polymer matrix. On the other hand, the muddled surface was due to adsorption of the polymer layers on the particles surface. The average size of the MgO particles was found to be approximately 6–11 nm for 10 wt% as the particles size slightly decreased with increasing magnesia content. Moreover, EDX measurements for the solid chitosan-MgO nanocomposite confirmed the presence of magnesia within the hybrid film, as shown in (Figure 4).
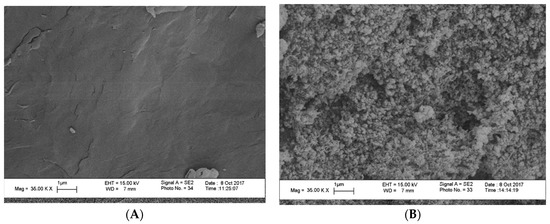
Figure 3.
FESEM images of chitosan (A), and chitosan-MgO 10 wt% (B).
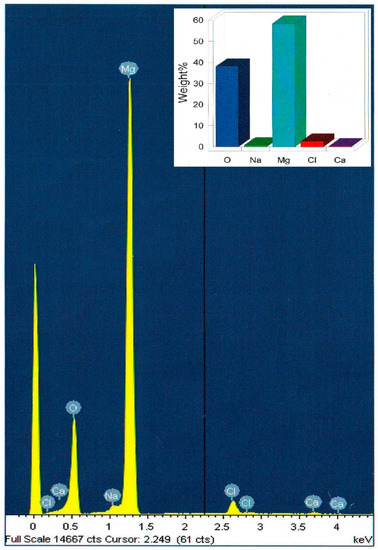
Figure 4.
EDX spectra of the 10 wt% chitosan-MgO nanocomposite hybrid film. Inset is the quantitative result.
3.2. Optimal Catalyst Loading
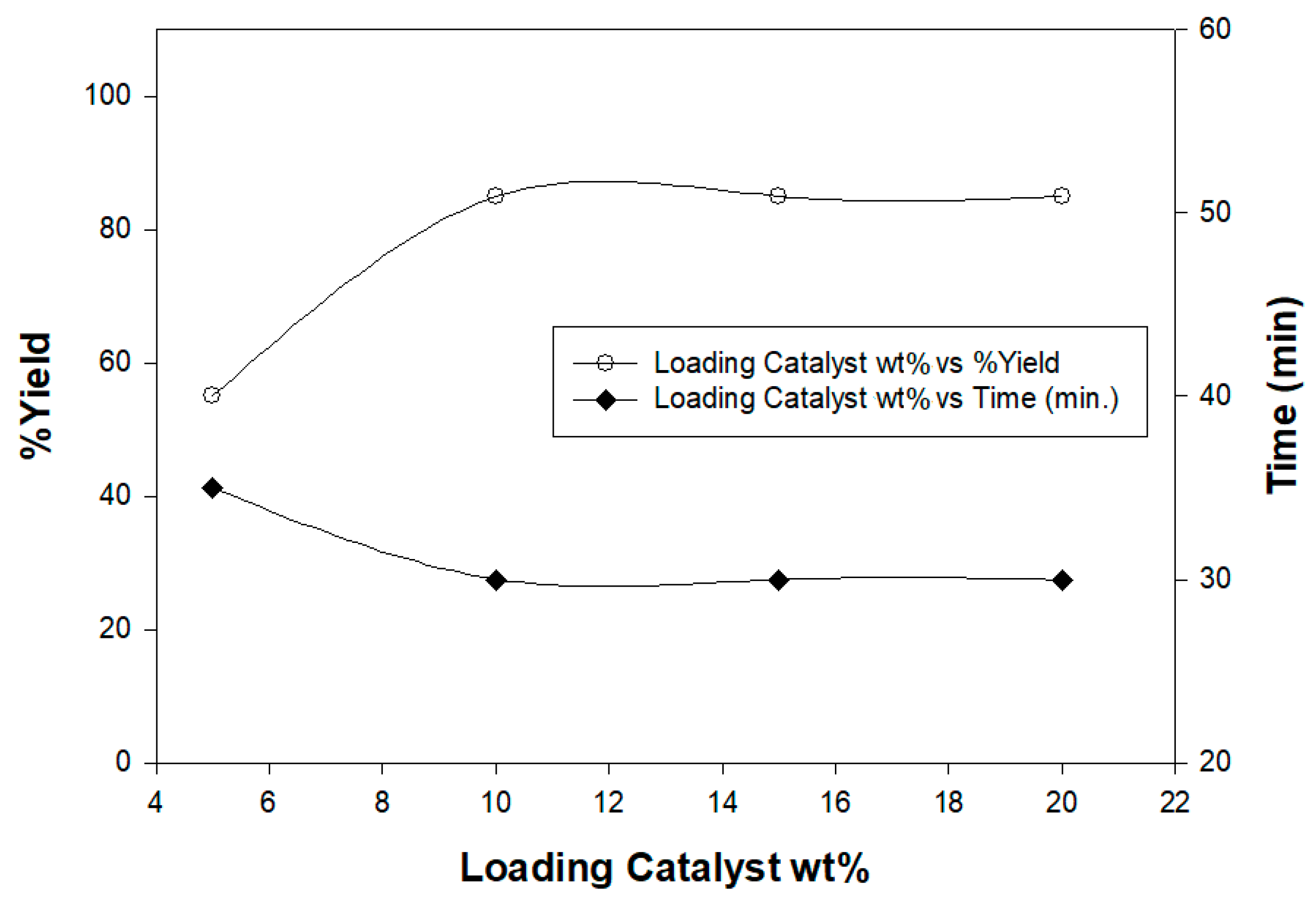
In order to estimate the proper catalyst loading, a model irradiation reaction of ethylidinethiosemicarbazide 1 with α-keto hydrazonoyl chlorides 2a in dioxane, and in the presence of 1, 5, 10, 15, and 20 wt% of chitosan-MgO nanocomposites as basic catalysts, under the same conditions was conducted affording the respective hydrazonothiazoles 4a (Figure 5). The results showed that the optimal catalyst loading was 10 wt% Moreover, the catalyst was reused four time and the results also showed that the biocatalyst could be reused as such without significant loss in its catalytic activity (Table 1).
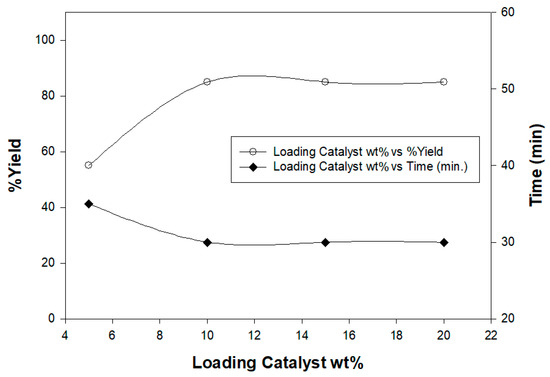
Figure 5.
Optimization of the chitosan graft copolymer as a basic catalyst.

Table 1.
Recyclability of the chitosan graft copolymer as basic catalyst.
3.3. Synthesis of Thiazoles and [1,3,4]thiadiazoles Using Cs-MgO Nanocomposite
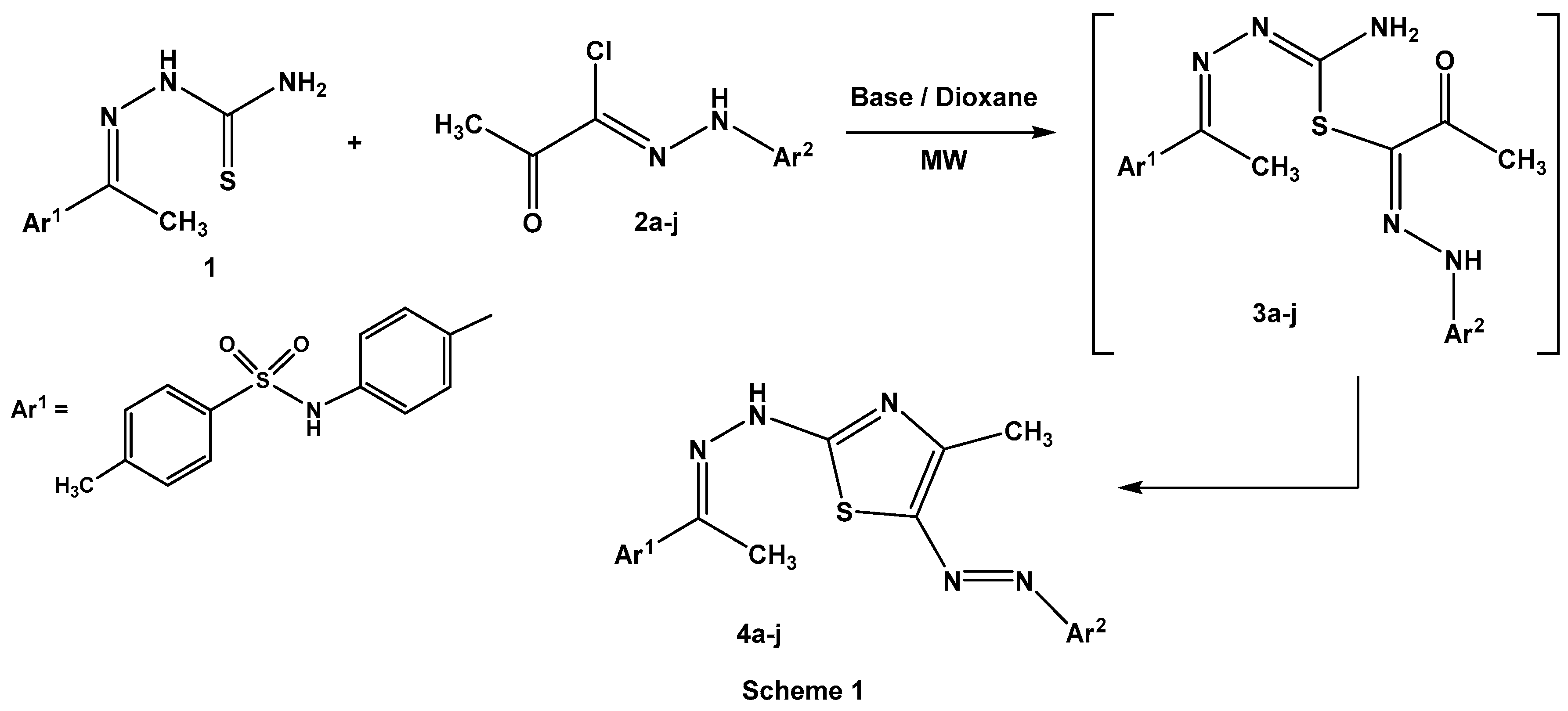
Irradiation of thiosemicarbazone 1 with 2-oxo-N-arylpropanehydrazonoyl chlorides 2a–j in dioxane, in the presence of triethylamine or the chitosan-MgO nanocomposite as a basic catalyst, furnished 4-methyl-5-arylazo-2-hydrazonothiazoles 4a–j (Scheme 1). At the outset, identification of the best basic catalyst (triethylamine or chitosan-MgO nanocomposite) was examined under microwave irradiations (Table 2).
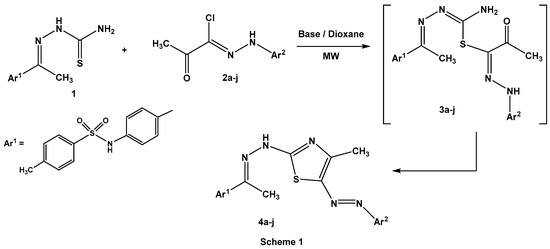
Scheme 1.
Synthesis of hydrazonothiazoles 4a–j.

Table 2.
Effect of the nature of basic catalyst on the yield of the products 4a–j.
As shown in Table 2, the reaction proceeded smoothly with different substituents on the aromatic benzene ring of hydrazonoyl chlorides 2a–j. Also, the chitosan-MgO nanocomposite was a more efficient basic catalyst than triethylamine under microwave irradiation.
Elucidation of 2-hydrazonothiazoles 4a–j structures was based on spectral data and elemental analyses. In the IR spectra, two absorption bands in the range of υ = 3212–3268 cm−1 and 1578–1600 cm−1 were revealed owing to the presence of (N–H) and (C=N) groups, respectively. Also, the sulfonamide group (SO2NH) showed asymmetric and symmetric stretching signals at υ = 1336–1379 cm−1 and 1136–1161 cm−1, respectively [22]. In 1H NMR spectra two methyl groups bordering to hydrazone moiety (CH3–C=N–NH) [23] and a thiazole ring [24] were observed as singlet signals at δ = 2.35–2.43 and 2.43–2.59 ppm, respectively, while the NH proton of the sulfonamide group [22] was resonated at δ = 10.43–10.92 ppm.
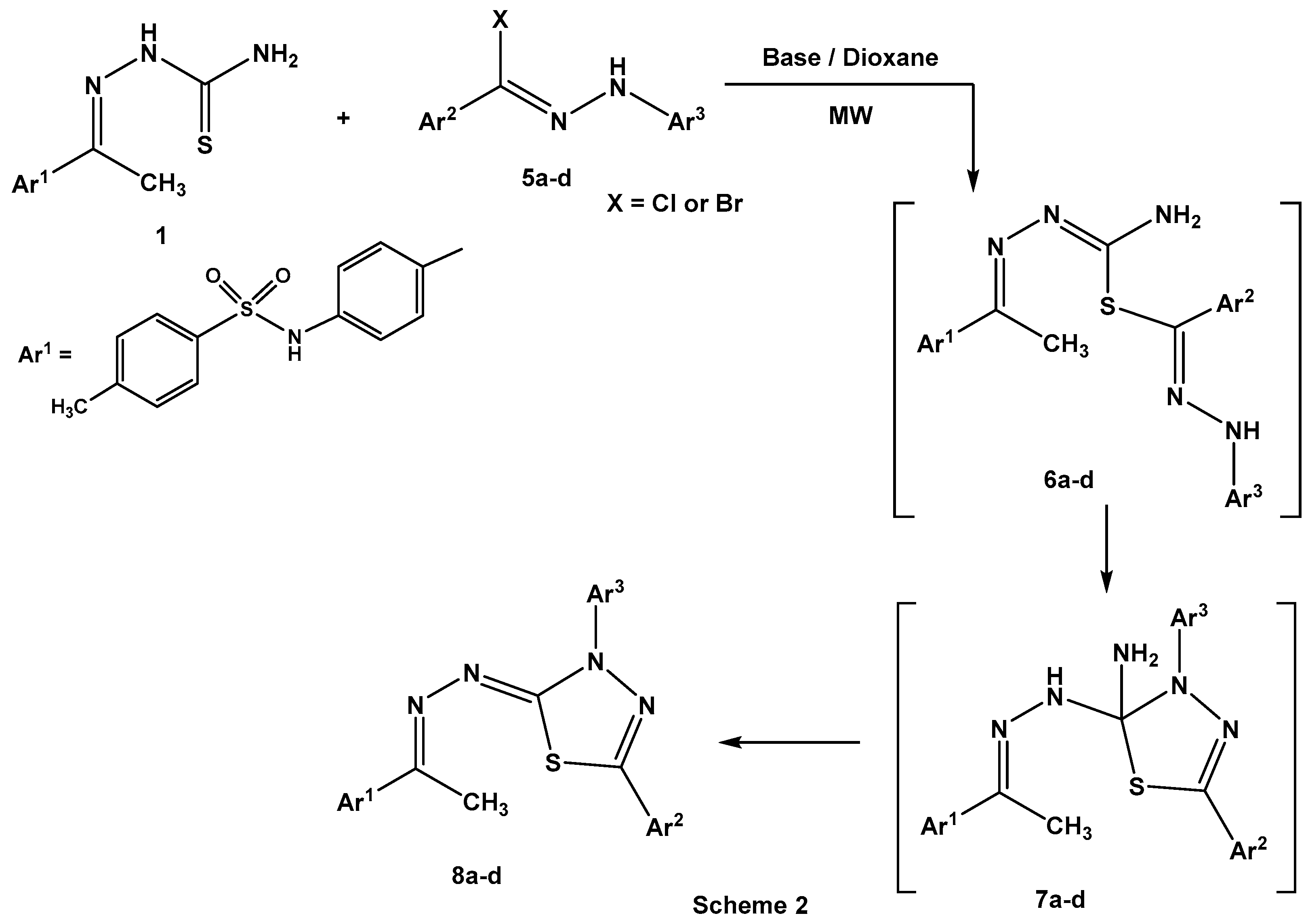
Establishing the experimental feasibility of the reaction of 1 with α-keto hydrazonoyl chlorides 2a–j directed our attention to use N-aryl arenecarbohydrazonoyl halides 5a–d, bereft of the carbonyl group. Thus, treatment of ethylidinethiosemicarbazide 1 with N-aryl arenecarbohydrazonoyl halides 5a–d under the same employed conditions proceeded smoothly to give 2-hydrazono[1,3,4] thiadiazoles 8a–d as the isolated products (Scheme 2). Also, the effect of the nature of the basic catalyst, such as triethylamine or the chitosan-MgO nanocomposite, on the percent yields of the isolated products 8a–d was investigated (Table 3).
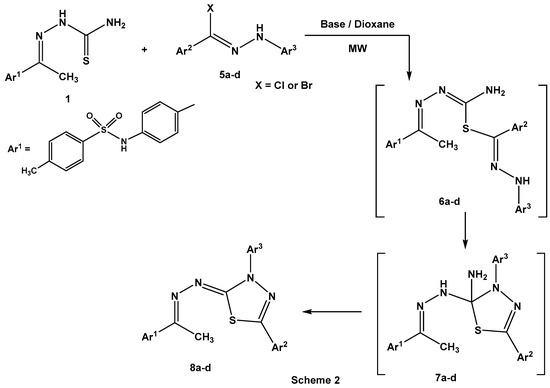
Scheme 2.
Synthesis of hydrazono[1,3,4]thiadiazoles 8a–d.

Table 3.
Effect of the nature of basic catalyst on the yield of the products 8a–d.
As shown in Table 3, the percentage yields of the products 8a–d, the chitosan-MgO nanocomposite as a basic catalyst prevailed over triethylamine under microwave irradiation. The characterization of 2-hydrazono[1,3,4]thiadiazoles 8a–d was consistent with spectral data (IR, 1H NMR, 13C NMR, and MS (Mass spectroscopy)) and elemental analyses (see Supplementary Materials). As shown in Scheme 2, the reaction proceeded through nucleophilic displacement of the thiol group to the halogen atom to give S-alkylated intermediate products 6a–d [25]. Intramolecular Michael addition [26] of the NH group into the electrophilic carbon atom of (C=N-N=) for intermediates 6a–d led to the formation of cycloadducts 7a–d. Elimination of ammonia from the latter intermediates 7a–d gave the final products 8a–d (Scheme 2).
4. Conclusions
Recently, nanoparticles (NPs) have been developed as promising candidates in various applications due to their unique properties. In this article, a chitosan-MgO nanocomposite (as a green recyclable biocatalyst) was prepared and well-characterized using FTIR, FESEM, and EDX spectra. The average size of the MgO particles was found to be approximately 6–11 nm for 10 wt% and it was found that the particles’ size slightly decreased with increasing magnesia content. This nanocomposite was then used successfully as a heterogeneous basic catalyst for the synthesis of two series of hydrazonothiazoles and hydrazono [1,3,4] thiadiazoles, with sulfonamide moiety, in a comparative study with triethylamine (as a traditional catalyst). In addition to the preferable green impact, the acquired results showed that the chitosan-MgO nanocomposite was a more powerful catalyst in these reactions as compared to triethylamine. The obvious catalytic potency of the chitosan-MgO nanocomposite was attributed to the obtained nanosized MgO and the synergistic effect that is created by the combination of the basic nature of both MgO and chitosan. Moreover, the nanocatalyst could be easily recovered and reused many times without loss in its catalytic activity. Finally, the biopolymer chitosan-metal oxide combination is a promising hybrid nanocomposite that deserves to be explored in many organic transformations.
Supplementary Materials
The following are available online at http://www.mdpi.com/2079-4991/8/11/928/s1.
Author Contributions
S.M.R. suggested the plan, interpreted the results, and wrote the chemistry part of the manuscript; K.D.K. prepared the chitosan-MgO nanocomposite, interpreted the results, performed the experiments, and wrote the other part of the manuscript; A.A. revised the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Babaie, M.; Sheibani, H. Nanosized magnesium oxide as a highly effective heterogeneous base catalyst for the rapid synthesis of pyranopyrazoles via a tandem four-component reaction. Arab. J. Chem. 2011, 4, 159–162. [Google Scholar] [CrossRef]
- Kumar, D.; Reddy, V.B.; Mishra, B.G.; Rana, R.K.; Nadagouda, M.N.; Varma, R.S. Nanosized magnesium oxide as catalyst for the rapid and green synthesis of substituted 2-amino-2-chromenes. Tetrahedron 2007, 63, 3093–3097. [Google Scholar] [CrossRef]
- Mirzaei, H.; Davoodnia, A. Microwave Assisted Sol-Gel Synthesis of MgO Nanoparticles and Their Catalytic Activity in the Synthesis of Hantzsch 1,4-Dihydropyridines. Chin. J. Catal. 2012, 33, 1502–1507. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Khalil, K.D.; Meier, H.; Kolshorn, H.; Elnagdi, M.H. Chitosan as heterogeneous catalyst in Michael additions: The reaction of cinnamonitriles with active methylene Moieties and phenols. Arkivoc 2008, 288–301. [Google Scholar] [CrossRef]
- Khalil, K.D.; Al-Matar, H.M.; Elnagdi, M.H. Chitosan as eco-friendly heterogeneous catalyst in Michael type additions: Simple and efficient route to pyridones and phthalazines. Eur. J Chem. 2010, 1, 252–258. [Google Scholar] [CrossRef]
- Khalil, K.D.; Al-Matar, H.M. Chitosan based heterogeneous catalyses: 4-Vinylpyridine grafted chitosan as catalyses for Michael additions and alkylpyridazinyl carbonitrile oxidation. Molecules 2013, 18, 5288–5305. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.S.; Praveen, K.S.; Sushil, K.G.; Dau, D.A. Chitosan: An efficient, reusable, and biodegradable catalyst for green synthesis of heterocycles. Ind. Eng. Chem. Res. 2014, 53, 2085–2091. [Google Scholar] [CrossRef]
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Studies on Magnesium Oxide Reinforced Chitosan Bionanocomposite Incorporated with Clove Oil for Active Food Packaging Application. Int. J. Polym. Mater. Biopolym. Mater. 2014, 63, 733–740. [Google Scholar] [CrossRef]
- Basumallick, S.; Santra, S. Chitosan coated copper-oxide nano particles: A novel electro-catalyst for CO2 reduction. RSC Adv. 2014, 4, 63685–63690. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Zablotskaya, A.; Segal, I.; Geronikaki, A.; Eremkina, T.; Belyakov, S.; Petrova, M.; Shestakova, I.; Zvejniece, V.; Nikolajeva, V. Synthesis, physicochemical characterization, cytotoxicity, antimicrobial, anti-inflammatory and psychotropic activity of new N-[1,3-(benzo)thiazol-2-yl]-ω-[3,4-dihydroisoquinolin-2(1H)-yl]alkanamides. Eur. J. Med. Chem. 2013, 70, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Djukicm, M.; Fesatidou, M.; Xenikakis, I.; Geronikaki, A.; Angelova, V.T.; Savic, V.; Pasic, M.; Krilovic, B.; Djukic, D.; Gobeljic, B.; et al. In vitro antioxidant activity of thiazolidinone derivatives of 1,3-thiazole and 1,3,4-thiadiazole. Chem. Biol. Interact. 2018, 286, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. [1,3,4]Thiadiazole: Synthesis, reactions, and applications in medicinal, agriculture, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Riyadh, S.M.; El-Motairi, S.A.; Ahmed, H.E.A.; Khalil, K.D.; Habib, E.E. Synthesis, Biological Evaluation, and Molecular Docking of Novel Thiazoles and [1,3,4]Thiadiazoles Incorporating Sulfonamide Group as DHFR Inhibitors. Chem. Biodiver. 2018, 15, e1800231. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.M.H.; Gomha, S.M.; Farghaly, T.A.; Abdalla, M.M. Synthesis of New Thiazole Derivatives as Antitumor Agents. Curr. Org. Synth. 2016, 13, 456–465. [Google Scholar] [CrossRef]
- Al-Bogami, A.S.; Saleh, T.S.; Mekky, A.E.M.; Shaaban, M.R. Microwave assisted regioselective synthesis and 2D-NMR studies of novel azoles and azoloazines utilizing fluorine-containing building Blocks. J. Mol. Struct. 2016, 1121, 167–179. [Google Scholar] [CrossRef]
- Eweiss, N.F.; Osman, A. Synthesis of Heterocycles Part II. New Routes to Acetylthiadiazolines and Alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Mosselhi, M.A.N.; Abdallah, M.A.; Mohamed, Y.F.; Shawali, A.S. Synthesis and Tautomeric Structure of 7-Arylhydrazono-7H-[1,2,4]Triazolo[3,4-b][1,3,4]Thiadiazines. Phosphorous Sulfur Silicon 2002, 177, 487–496. [Google Scholar] [CrossRef]
- Laude, B.; Soufiaoui, M.; Arriau, J. Cycloadditions dipolaires-1,3 II. Addition des diarylnitrilimines au N-methylindole. Etude experimentale et essai d’interpretation. J. Heterocycl. Chem. 1977, 14, 1183–1190. [Google Scholar] [CrossRef]
- Wolkoff, P. A New Method of Preparing Hydrazonyl Halides. Can. J. Chem. 1975, 53, 1333–1335. [Google Scholar] [CrossRef]
- El Kadib, A.; Primo, A.; Molvinger, K.; Boumina, M.; Brunel, D. Nanosized vanadium, tungsten, and molybdenium oxide clusters grown in porous chitosan microspheres as promising hybrid materials for selective alcohol oxidation. Chem. A Eur. J. 2011, 17, 7940–7946. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.R.; Bahekar, S.P.; Agrawal, A.R.; Sarode, P.B.; Chandak, H.S. Cascade Michael-Aldol reaction: Efficient annulation of sulfonamide chalcones into novel cyclohexenones under solvent-free conditions. Arkivoc 2016, 227–245. [Google Scholar] [CrossRef]
- De Oliveira Cardoso, M.V.; de Siqueira, L.R.P.; de Silva, E.B.; Costa, L.B.; Hernandes, M.Z.; Rabello, M.M.; Ferreira, R.S.; de Cruz, L.F.; Moreira, D.R.M.; Pereira, V.R.A.; et al. 2-Pyridyl thiazoles as novel anti-Trypanosoma cruzi agents: Structural design, synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2014, 86, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Rostom, S.A.F.; Faidallah, H.M.; Radwan, M.F.; Badr, M.H. Bifunctional ethyl 2-amino-4-methylthiazole-5-carboxylate derivatives: Synthesis and in vitro biological evaluation as antimicrobial and anticancer agents. Eur. J. Med. Chem. 2014, 76, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Geies, A.A.; Kamal-Eldeen, A.M.; Abdelhafez, A.A.; Gaber, A.M. Synthesis of some thiazolo[3,2-a]pyrimidines. Phosphorous Sulfur Silicon Relat. Elem. 1991, 56, 87–93. [Google Scholar] [CrossRef]
- Ranu, B.C.; Banerjee, S. Ionic Liquid as Catalyst and Reaction Medium. The Dramatic Influence of a Task-Specific Ionic Liquid, [bmIm]OH, in Michael Addition of Active Methylene Compounds to Conjugated Ketones, Carboxylic Esters, and Nitriles. Org. Lett. 2005, 7, 3049–3052. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).