Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO
2.3. Synthesis of MIL-53(Al)
2.4. Synthesis of MIL-53(Al)-GO Nanocomposites
2.5. Adsorbent Characterization
2.6. Adsorption Experiments
3. Results and Discussion
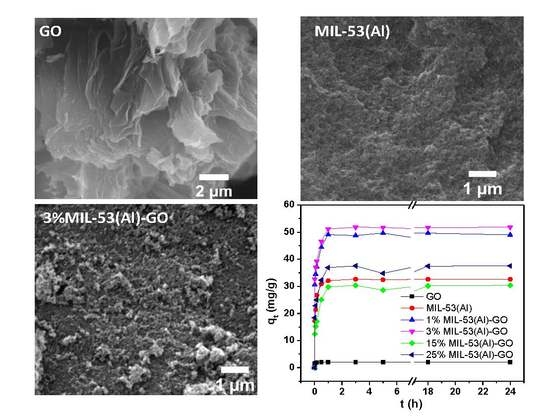
3.1. Nanomaterial Characterization
3.2. Adsorption Kinetics
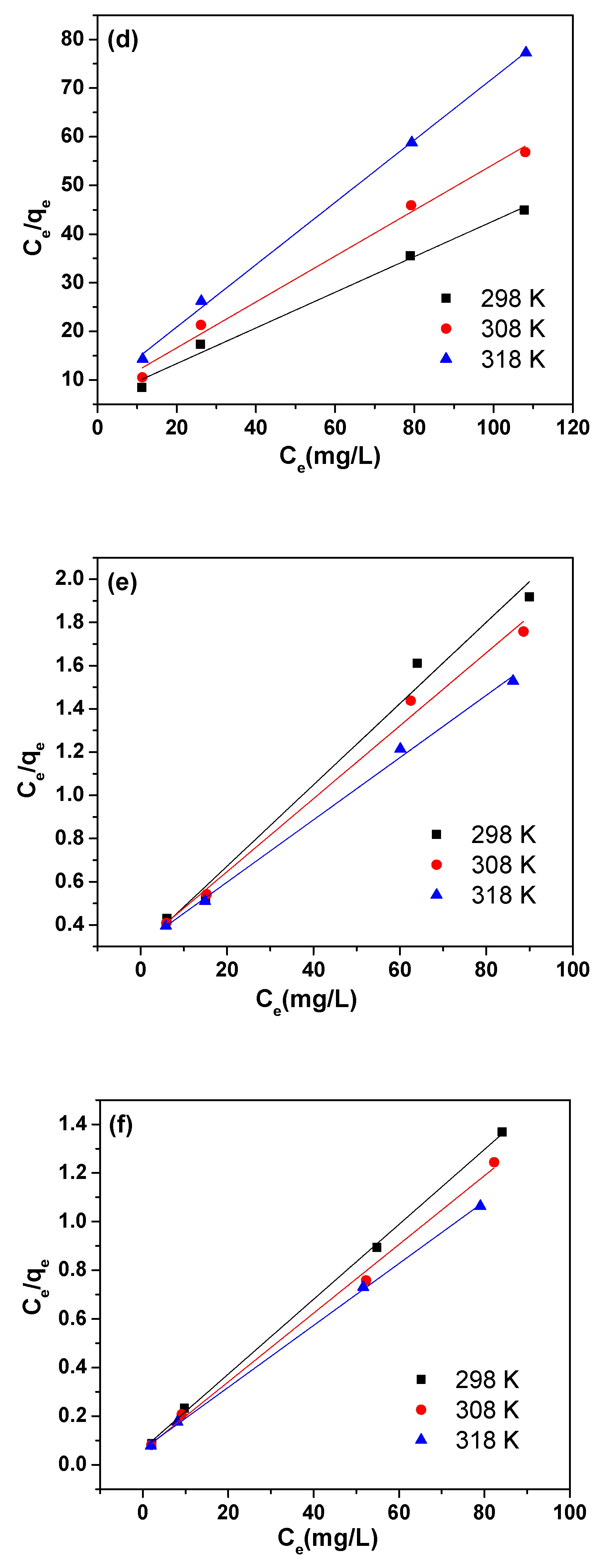
3.3. Adsorption Isotherms
3.4. Effect of pH on As(III) Adsorption
3.5. Effect of Initial As(III) Concentration on As(III) Adsorption
3.6. Effect of Adsorbent Dosage on As(III) Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Moradpour, T.; Van Hecke, K. A new 3D cobalt (II) metal-organic framework nanostructure for heavy metal adsorption. Inorg. Chim. Acta 2015, 430, 261–267. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Masoomi, M.Y.; Yamini, Y.; Morsali, A. Application of mechanosynthesized azine-decorated zinc(II) metal–organic frameworks for highly efficient removal and extraction of some heavy-metal ions from aqueous samples: A comparative study. Inorg. Chem. 2015, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Ke, Y.C.; Guo, D.; Guo, H.X.; Chen, J.H.; Weng, W. Facile fabrication of cauliflower-like MIL-100(Cr) and its simultaneous determination of Cd2+, Pb2+, Cu2+ and He2+ from aqueous solution. Sens. Actuators B 2015, 216, 504–510. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.M.; Petit, C. Towards the use of metal-organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Dominguez-Vargas, J.R.; Olivares-Marin, F.J.; de Heredia, J.B. On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. J. Hazard. Mater. 2010, 177, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, P.; Caroli, S.; Caracciolo, A.B. Pharmaceuticals as priority water contaminants. Toxicol. Environ. Chem. 2010, 92, 549–565. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Remarkable adsorptive removal of nitrogen-containing compounds from a model fuel by a graphene oxide/MIL-101 composite through a combined effect of improved porosity and hydrogen bonding. J. Hazard. Mater. 2016, 314, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P. Epidemiology of environmental and occupational cancer. Oncogene 2004, 23, 6392–6403. [Google Scholar] [CrossRef] [PubMed]
- Yang, M. A current global view of environmental and occupational cancers. J. Environ. Sci. Health C 2011, 29, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Hei, S.T.; Jin, Y.; Zhang, F.M. Fabrication of gamma-Fe2O3 nanoparticles by solid-state thermolysis of a metal-organic framework, MIL-100(Fe), for heavy metal ions removal. J. Chem. 2014, 6, 546956. [Google Scholar] [CrossRef]
- Jones, F.T. A broad view of arsenic. Poult. Sci. 2007, 86, 2–14. [Google Scholar] [CrossRef]
- Tuutijärvi, T.; Lu, J.; Sillanpää, M.; Chen, G. As(V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Closer look at As(III) and As(V) adsorption onto ferrihydrite under competitive conditions. Langmuir 2014, 30, 11110–11116. [Google Scholar] [CrossRef]
- Baba, Y.; Iwakuma, M.; Nagami, H. Extraction Mechanism for copper(II) with 2-hydroxy-4-n-octyloxybenzophenone oxime. Ind. Eng. Chem. Res. 2002, 41, 5835–5841. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Lee, J.-U.; Moon, S.-H.; Kim, K.-W. Competitive adsorption characteristics of Co2+, Ni2+, and Cr3+ by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 2004, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.M.; Teng, S.P.; Teng, T.T.; Mohd Omar, A.K. Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions. Water Qual. Res. J. Can. 2009, 44, 174–182. [Google Scholar] [CrossRef]
- Khedr, M.G. Membrane methods in tailoring simpler, more efficient, and cost effective wastewater treatment alternatives. Desalination 2008, 222, 135–145. [Google Scholar] [CrossRef]
- Abid, M.F.; Zablouk, M.A.; Abid-Alameer, A.M. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran. J. Environ. Health Sci. Eng. 2012, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, R.; Aliyan, H.; Banavandi, R.S. Sunlight assisted photodecolorization of malachite green catalyzed by MIL-101/graphene oxide composites. Russ. J. Appl. Chem. 2015, 88, 169–177. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X. Adsorption of graphene for the removal of inorganic pollutants in water purification: A review. Adsorption 2014, 20, 713–727. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Song, H.; Zhang, L.; Wan, X.; Tang, Y.; Lv, Y. SiO2/graphene composite for highly selective adsorption of Pb(II) ion. J. Colloid Interface Sci. 2012, 369, 381–387. [Google Scholar] [CrossRef]
- Bae, J.; Lee, E.J.; Jeong, N.C. Metal coordination and metal activation abilities of commonly unreactive chloromethanes toward metal-organic frameworks. Chem. Commun. 2018, 54, 6458. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Zhang, L. Hydrogen storage in metal–organic frameworks. Adv. Mater. 2010, 22, E117–E130. [Google Scholar] [CrossRef] [PubMed]
- Loera-Serna, S.; Zarate-Rubio, J.; Medina-Velazquez, D.Y.; Zhang, L.; Ortiz, E. Encapsulation of urea and caffeine in Cu3(BTC)2 metal–organic framework. Surf. Innov. 2016, 4, 76–87. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.H. Desorption of dimethylformamide from Zn4O(C8H4O4)3 framework. Appl. Surf. Sci. 2011, 257, 3392–3398. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.H. Observation of ZnO nanoparticles outside pores of nano Zn4O(C8H4O4)3 metal–organic framework. Phys. Lett. A 2011, 375, 1514–1517. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Zhang, C.; Yu, Q.; Zhao, Z.; Niu, X.; Sun, X.; Liu, Y.; Ma, L.; Li, Z. Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv. 2017, 7, 16273–16281. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A lanthanide metal-organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants. RSC Adv. 2016, 6, 11570–11576. [Google Scholar] [CrossRef]
- Ahmed, I.; Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive denitrogenation of model fuels with porous metal-organic framework (MOF) MIL-101 impregnated with phosphotungstic acid: Effect of acid site inclusion. J. Hazard. Mater. 2013, 250–251, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Surface Decoration of amino-functionalized metal–organic framework/graphene oxide composite onto polydopamine-coated membrane substrate for highly efficient heavy metal removal. ACS Appl. Mater. Interfaces 2017, 9, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yadian, B.; Wu, R.; Long, Y.; Zhou, K.; Zhu, B.; Huang, Y. Structure stability of metal-organic framework MIL-53 (Al) in aqueous solutions. Int. J. Hydrog. Energy 2013, 38, 16710–16715. [Google Scholar] [CrossRef]
- Dey, R.S.; Hajra, S.; Sahu, R.K.; Raj, C.R.; Panigrahi, M.K. A rapid room temperature chemical route for the synthesis of graphene: Metal-mediated reduction of graphene oxide. Chem. Commun. 2012, 48, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Ricco, R.; Konstas, K.; Styles, M.J.; Richardson, J.J.; Babarao, R.; Suzuki, K.; Scopece, P.; Falcaro, P. Lead(II) uptake by aluminium based magnetic framework composites (MFCs) in water. J. Mater. Chem. A 2015, 3, 19822–19831. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Vinh-Thang, H.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Polymer functionalization to enhance interface quality of mixed matrix membranes for high CO2/CH4 gas separation. J. Mater. Chem. A 2015, 3, 15202–15213. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2014, 89, 196–205. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Zeng, G.; Chen, X.; Wang, H.; zhang, L.; Shao, J. Enhanced adsorptive removal of p-nitrophenol from water by aluminum metal-organic framework/reduced graphene oxide composite. Sci. Rep. 2016, 6, 25638. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, X.; Li, Y. Controlled growth of dense and ordered metal-organic framework nanoparticles on graphene oxide. Chem. Commun. 2015, 51, 3874–3877. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, A.; Hou, K.; Liu, M.; Wang, Y.; Song, C.; Zhang, G.; Guo, X. Size- and morphology-controlled NH2-MIL-53(Al) prepared in DMF-water mixed solvents. Dalton Trans. 2013, 42, 13698–13705. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-benzenetricarboxylic metal–organic coordination polymers prepared by solvothermal method and their application in efficient As(V) removal from aqueous solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Manju, G.N.; Raji, C.; Anirudhan, T.S. Evaluation of coconut husk carbon for the removal of arsenic from water. Water Res. 1998, 32, 3062–3070. [Google Scholar] [CrossRef]
- Lenoble, V.; Bouras, O.; Deluchat, V.; Serpaud, B.; Bollinger, J.-C. Arsenic adsorption onto pillared clays and iron oxides. J. Colloid Interfacce Sci. 2002, 255, 52–58. [Google Scholar] [CrossRef]
- Bang, S.; Patel, M.; Lippincott, L.; Meng, X. Removal of arsenic from groundwater by granular titanium dioxide adsorbent. Chemosphere 2005, 60, 389–397. [Google Scholar] [CrossRef]
- Chen, W.; Parette, R.; Zou, J.; Cannon, F.S.; Dempsey, B.A. Arsenic removal by iron-modified activated carbon. Water Res. 2007, 41, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef]
- You, L.; Wu, Z.; Kim, T.; Lee, K. Kinetics and thermodynamics of bromophenol blue adsorption by a mesoporous hybrid gel derived from tetraethoxysilane and bis(trimethoxysilyl)hexane. J. Colloid Interface Sci. 2006, 300, 526–535. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Li, Z.; Zhu, M.; Li, F. Characteristics of arsenate removal from water by metal-organic frameworks (MOFs). Water Sci. Technol. 2014, 70, 1391–1397. [Google Scholar] [CrossRef]
- Leng, L.; Yuan, X.; Huang, H.; Shao, J.; Wang, H.; Chen, X.; Zeng, G. Bio-char derived from sewage sludge by liquefaction: Characterization and application for dye adsorption. Appl. Surf. Sci. 2015, 346, 223–231. [Google Scholar] [CrossRef]
- Payne, K.B.; Abdel-Fattah, T.M. Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: Effects of pH, temperature, and ionic strength. J. Environ. Sci. Health A 2005, 40, 723–749. [Google Scholar] [CrossRef]
- Ansari, R.; Sadegh, M. Application of activated carbon for removal of arsenic ions from aqueous solutions. E-J. Chem. 2007, 4, 103–108. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Z.; Guo, Y.; Qiu, Y.; Zhao, J. Facile synthesis of mesoporous Ce-Fe bimetal oxide and its enhanced adsorption of arsenate from aqueous solutions. J. Colloid Interface Sci. 2013, 398, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Mondal, P.; Majumder, C.B.; Mohanty, B. Effects of adsorbent dose, its particle size and initial arsenic concentration on the removal of arsenic, iron and manganese from simulated ground water by Fe3+ impregnated activated carbon. J. Hazard. Mater. 2008, 150, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Mohamad Moosavi, S.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, selective heavy metal removal from water by a metal–organic framework/polydopamine composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef]





| Model | Parameters | GO | MIL-53(Al) | 1% MIL-53(Al)-GO | 3% MIL-53(Al)-GO | 15% MIL-53(Al)-GO | 25% MIL-53(Al)-GO |
|---|---|---|---|---|---|---|---|
| Pseudo-first-order | qe,exp (mg/g) | 2.05 | 32.65 | 49.73 | 51.80 | 32.45 | 37.55 |
| k1 (1/h) | 0.18 | 0.23 | 0.18 | 0.26 | 0.08 | 0.23 | |
| qe,cal (mg/g) | 0.17 | 1.70 | 4.57 | 3.53 | 9.29 | 9.39 | |
| R2 | 0.323 | 0.289 | 0.311 | 0.447 | 0.345 | 0.561 | |
| Pseudo-second-order | k2 (g/mg·h−1) | 14.19 | 1.40 | 0.73 | 0.62 | 0.28 | 0.26 |
| qe,cal (mg/g) | 2.04 | 32.64 | 49.31 | 51.84 | 30.14 | 37.34 | |
| R2 | 0.999 | 0.999 | 0.998 | 0.999 | 0.996 | 0.998 | |
| Intra-particle diffusion | K1d (mg/(g·h1/2)) | 0.48 | 17.03 | 21.54 | 21.31 | 20.79 | 21.31 |
| C1 | 1.63 | 17.04 | 28.26 | 30.54 | 9.37 | 16.29 | |
| (R1)2 | 0.768 | 0.842 | 0.990 | 0.989 | 0.988 | 0.989 | |
| K2d (mg/(g·h1/2)) | 0.01 | 0.09 | 0.08 | 0.10 | 0.07 | 0.14 | |
| C2 | 1.99 | 32.28 | 48.78 | 51.32 | 29.37 | 36.22 | |
| (R2)2 | 0.227 | 0.441 | 0.035 | 0.267 | 0.012 | 0.028 |
| Adsorbent | Temperature (K) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | RL | R2 | 1/n | KF (mg/g)·(L/mg)1/n | R2 | ||
| GO | 298 | 2.72 | 0.06 | 0.22 | 0.988 | 0.28 | 0.65 | 0.960 |
| 308 | 2.12 | 0.07 | 0.19 | 0.988 | 0.26 | 0.56 | 0.971 | |
| 318 | 1.56 | 0.08 | 0.18 | 0.998 | 0.25 | 0.43 | 0.993 | |
| MIL-53(Al) | 298 | 53.16 | 0.06 | 0.22 | 0.981 | 0.40 | 7.82 | 0.877 |
| 308 | 59.21 | 0.05 | 0.26 | 0.991 | 0.44 | 7.36 | 0.943 | |
| 318 | 69.39 | 0.05 | 0.26 | 0.996 | 0.48 | 6.92 | 0.959 | |
| 3% MIL-53(Al)-GO | 298 | 64.97 | 0.24 | 0.07 | 0.999 | 0.26 | 21.08 | 0.944 |
| 308 | 70.77 | 0.24 | 0.07 | 0.996 | 0.28 | 21.45 | 0.938 | |
| 318 | 78.55 | 0.20 | 0.08 | 0.999 | 0.29 | 22.06 | 0.949 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite. Nanomaterials 2018, 8, 1062. https://doi.org/10.3390/nano8121062
Chowdhury T, Zhang L, Zhang J, Aggarwal S. Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite. Nanomaterials. 2018; 8(12):1062. https://doi.org/10.3390/nano8121062
Chicago/Turabian StyleChowdhury, Tonoy, Lei Zhang, Junqing Zhang, and Srijan Aggarwal. 2018. "Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite" Nanomaterials 8, no. 12: 1062. https://doi.org/10.3390/nano8121062
APA StyleChowdhury, T., Zhang, L., Zhang, J., & Aggarwal, S. (2018). Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite. Nanomaterials, 8(12), 1062. https://doi.org/10.3390/nano8121062