Development and Characterization of Solid Lipid Nanoparticles Loaded with a Highly Active Doxorubicin Derivative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of SQ-Dox and Preparation of SQ-Dox NAs
2.2.2. Preparation SQ-Dox-Loaded SLNs
2.2.3. NA and SLN Characterization
2.2.3.1. Size and Zeta Potential Measurements
2.2.3.2. Differential Scanning Calorimetry
2.2.3.3. Atomic Force Microscopy
2.2.3.4. SQ-Dox Stability Evaluation: HPLC Analysis
2.2.4. In Vitro Biological Evaluations
2.2.4.1. Cell Cultures
2.2.4.2. Cell Viability Assay
2.2.4.3. Colony-Forming Assay
2.2.4.4. Cell Death Analysis
2.2.5. Statistical Analysis
3. Results
3.1. NA and SLN Preparation and Characterization
3.2. In Vitro Evaluations
3.2.1. SQ-Dox NA and SQ-Dox SLN1 Inhibition in Cell Proliferation Tests
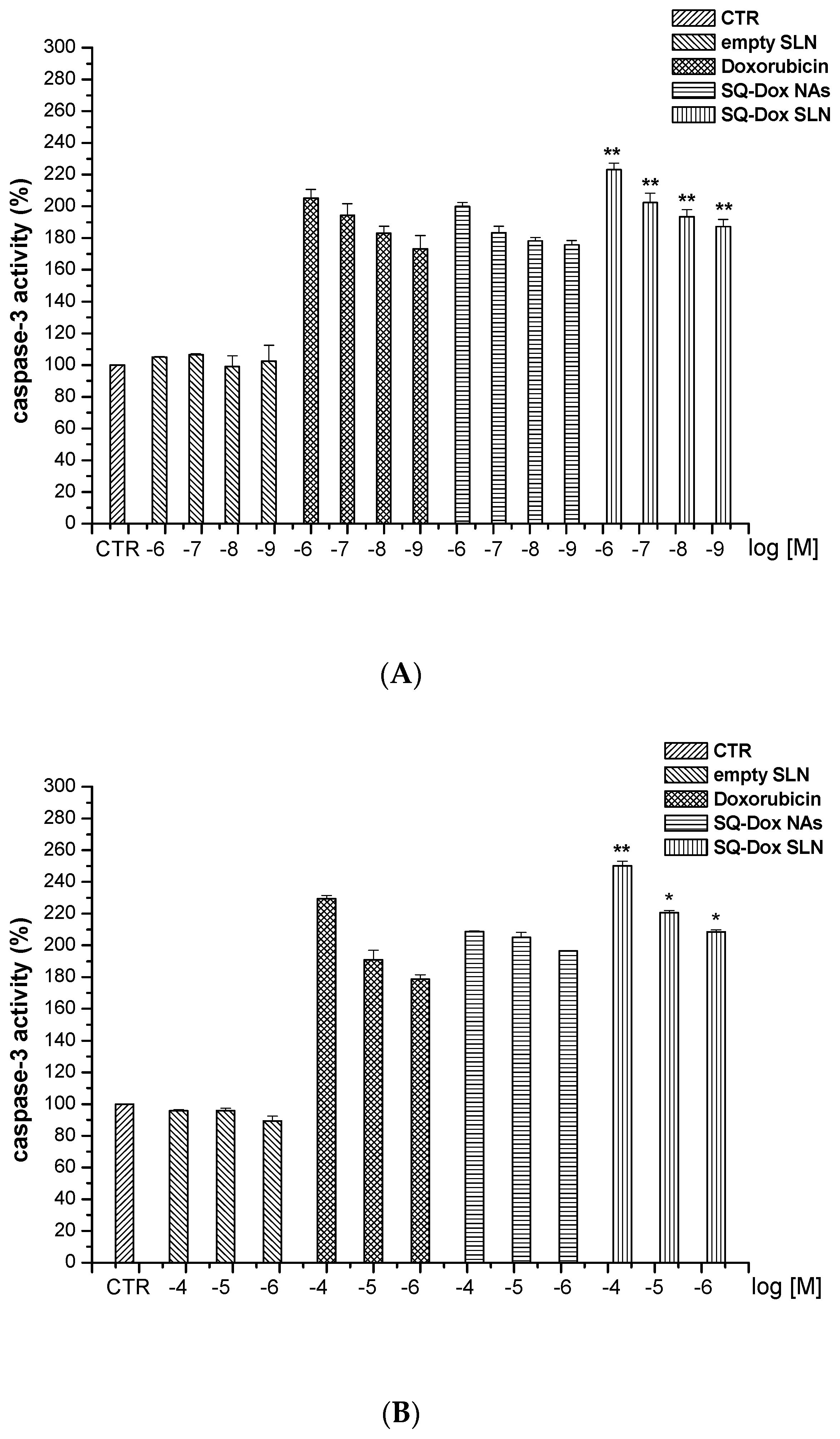
3.2.2. SQ-Dox NAs and SQ-Dox SLN1 Increase Caspase-3 Activity In Vitro
3.2.3. SQ-Dox NAs and SQ-Dox SLN1; Levels of Annexin-V
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Herman, E.H.; Lipshultz, S.E.; Minotti, G.; Sarvazyan, N.; Sawyer, D.B. Anthracycline cardiotoxicity: From bench to bedside. J. Clin. Oncol. 2008, 26, 3777–3784. [Google Scholar] [CrossRef] [PubMed]
- Nadas, J.; Sun, D. Anthracyclines as effective anticancer drugs. Expert Opin. Drug Discov. 2006, 1, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Kubicka-Wołkowska, J.; Kędzierska, M.; Lisik-Habib, M.; Potemski, P. Skin toxicity in a patient with ovarian cancer treated with pegylated liposomal doxorubicin: A case report and review of the literature. Oncol. Lett. 2016, 12, 5332–5334. [Google Scholar] [CrossRef] [PubMed]
- Arpicco, S.; Dosio, F.; Stella, B.; Cattel, L. Anticancer prodrugs: An overview of major strategies and recent developments. Curr. Top. Med. Chem. 2011, 11, 2346–2381. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, A.; Dosio, F.; Mougin, J.; Ferrero, A.; Wack, S.; Reddy, L.H.; Weyn, A.-A.; Lepeltier, E.; Bourgaux, C.; Stella, B.; et al. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc. Natl. Acad. Sci. USA 2014, 111, E217–E226. [Google Scholar] [CrossRef] [PubMed]
- Sobot, D.; Mura, S.; Yesylevskyy, S.O.; Dalbin, L.; Cayre, F.; Bort, G.; Mougin, J.; Desmaële, D.; Lepetre-Mouelhi, S.; Pieters, G.; et al. Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nat. Commun. 2017, 8, 15678. [Google Scholar] [CrossRef] [PubMed]
- Sobot, D.; Mura, S.; Rouquette, M.; Vukosavljevic, B.; Cayre, F.; Buchy, E.; Pieters, G.; Garcia-Argote, S.; Windbergs, M.; Desmaële, D.; et al. Circulating Lipoproteins: A Trojan Horse Guiding Squalenoylated Drugs to LDL-Accumulating Cancer Cells. Mol. Ther. 2017, 25, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Patro, N.M.; Devi, K.; Pai, R.S.; Suresh, S. Evaluation of bioavailability, efficacy, and safety profile of doxorubicin-loaded solid lipid nanoparticles. J. Nanopart. Res. 2013, 15, 2124. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Anthracycline nano-delivery systems to overcome multiple drug resistance: A comprehensive review. Nano Today 2013, 8, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Chun, M.-K.; Kim, O.; Subedi, R.K.; Ahn, S.-G.; Yoon, J.-H.; Choi, H.-K. Doxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Trapani, A.; Denora, N.; Wang, F.; Gallo, J.M.; Trapani, G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin. Drug Deliv. 2009, 6, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Muntoni, E.; Chirio, D.; Peira, E.; Annovazzi, L.; Schiffer, D.; Mellai, M.; Riganti, C.; Salaroglio, I.C.; Lanotte, M.; et al. Solid lipid nanoparticles by coacervation loaded with a methotrexate prodrug: Preliminary study for glioma treatment. Nanomedicine 2017, 12, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.J.; Sekido, M.; Lee, R.J. A folate receptor-targeted lipid nanoparticle formulation for a lipophilic paclitaxel prodrug. Pharm. Res. 2004, 21, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Reis, S. Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways. Bioconj. Chem. 2017, 28, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Gallarate, M.; Peira, E.; Battaglia, L.; Serpe, L.; Trotta, M. Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J. Microencapsul. 2011, 28, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Irby, D.; Du, C.; Li, F. Lipid-Drug Conjugate for Enhancing Drug Delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Scholes, P.D.; Coombes, A.G.A.; Illum, L.; Davis, S.S.; Watts, J.F.; Ustariz, C.; Vert, M.; Davies, M.C. Detection and determination of surface levels of poloxamer and PVA surfactant on biodegradable nanospheres using SSIMS and XPS. J. Control. Release 1999, 59, 261–278. [Google Scholar] [CrossRef]
- Peira, E.; Chirio, D.; Battaglia, L.; Barge, A.; Chegaev, K.; Gigliotti, C.L.; Ferrara, B.; Dianzani, C.; Gallarate, M. Solid lipid nanoparticles carrying lipophilic derivatives of doxorubicin: Preparation, characterization, and in vitro cytotoxicity studies. J. Microencapsul. 2016, 33, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Serpe, L.; Foglietta, F.; Zara, G.P.; Giordano, S.; Peira, E.; Chirio, D.; Battaglia, L. Solid lipid nanoparticles loaded with fluorescent-labelled cyclosporine A: Anti-inflammatory activity in vitro. Protein Pept. Lett. 2014, 21, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.K.; Vuddanda, P.R.; Karunanidhi, P.; Singh, S.K.; Singh, S. Development and Evaluation of Solid Lipid Nanoparticles of Raloxifene Hydrochloride for Enhanced Bioavailability. Biomed. Res. Int. 2013, 2013, 584549. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, B.; Westesen, K. Thermoanalysis of the recrystallization process of melt-homogenized glyceride nanoparticles. Colloids Surf. B Biointerfaces 1994, 3, 159–175. [Google Scholar] [CrossRef]
- Hou, D.; Xie, C.; Huang, K.; Zhu, C. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials 2003, 24, 1781–1785. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264 (Suppl. C), 306–332. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Cavalli, R.; Trotta, M. Solid lipid nanoparticles produced through a coacervation method. J. Microencapsul. 2010, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Peira, E.; Chirio, D.; Muntoni, E.; Biasibetti, E.; Capucchio, M.T.; Valazza, A.; Panciani, P.P.; Lanotte, M.; et al. Solid lipid nanoparticles for potential doxorubicin delivery in glioblastoma treatment: Preliminary in vitro studies. J. Pharm. Sci. 2014, 103, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, G.; Stella, B.; Pastushenko, I.; Ricci, F.; Christodoulou, M.S.; Damia, G.; Mazza, D.; Arpicco, S.; Giannini, C.; Morosi, L.; et al. Heteronanoparticles by self-Assembly of Doxorubicin and Cyclopamine Conjugates. ACS Med. Chem. Lett. 2017, 8, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Reddy, L.H.; Othman, M.; Gillet, B.; Desmaële, D.; Zouhiri, F.; Dosio, F.; Gref, R.; Couvreur, P. Squalene based nanocomposites: A new platform for the design of multifunctional pharmaceutical theragnostics. ACS Nano 2011, 5, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Pili, B.; Reddy, L.H.; Bourgaux, C.; Lepêtre-Mouelhi, S.; Desmaële, D.; Couvreur, P. Liposomal squalenoyl-gemcitabine: Formulation, characterization and anticancer activity evaluation. Nanoscale 2010, 2, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Skommer, J.; Darzynkiewicz, Z. Flow cytometry-based apoptosis detection. Methods Mol. Biol. 2009, 559. [Google Scholar] [CrossRef]
- Mazzoni, A.; Trave, F. Cytoplasmic membrane cholesterol and doxorubicin cytotoxicity in drug-sensitive and multidrug-resistant human ovarian cancer cells. Oncol. Res. 1993, 5, 75–82. [Google Scholar] [PubMed]





| Samples | Mean Diameter (nm) ± S.E. | PDI | Zeta Potential (mV) ± S.E. | EE% ± S.E. | Mean Diameter (nm) ± S.E. | ||
|---|---|---|---|---|---|---|---|
| T = 15 d | T = 45 d | T = 72 h Cell Medium | |||||
| SQ-Dox NAs | 130 ± 17 | 0.086 | 12.12 ± 1.52 | - | 150 ± 7 | 160 ± 18 | 450 ± 35 |
| Empty SLNs | 272 ± 6 | 0.108 | −2.20 ± 0.84 | - | 280 ± 9 | 282 ± 12 | 270 ± 12 |
| SQ-Dox SLN1 | 400 ± 10 | 0.227 | 10.34 ± 2.47 | 85 ± 3 | 420 ± 5 | 394 ± 6 | 400 ± 7 |
| SQ-Dox SLN2 | 305 ± 6 | 0.200 | 2.57 ± 0.43 | 50 ± 3 | 348 ± 5 | 355 ± 5 | 320 ± 8 |
| Tonset (°C) | Tm (°C) | ΔH (KJ/g) | |
|---|---|---|---|
| SQ-Dox NAs | 148.09 | 153.47 | 141.460 |
| SQ-Dox SLN1 | 139.28 | 141.18 | 49.960 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stella, B.; Peira, E.; Dianzani, C.; Gallarate, M.; Battaglia, L.; Gigliotti, C.L.; Boggio, E.; Dianzani, U.; Dosio, F. Development and Characterization of Solid Lipid Nanoparticles Loaded with a Highly Active Doxorubicin Derivative. Nanomaterials 2018, 8, 110. https://doi.org/10.3390/nano8020110
Stella B, Peira E, Dianzani C, Gallarate M, Battaglia L, Gigliotti CL, Boggio E, Dianzani U, Dosio F. Development and Characterization of Solid Lipid Nanoparticles Loaded with a Highly Active Doxorubicin Derivative. Nanomaterials. 2018; 8(2):110. https://doi.org/10.3390/nano8020110
Chicago/Turabian StyleStella, Barbara, Elena Peira, Chiara Dianzani, Marina Gallarate, Luigi Battaglia, Casimiro Luca Gigliotti, Elena Boggio, Umberto Dianzani, and Franco Dosio. 2018. "Development and Characterization of Solid Lipid Nanoparticles Loaded with a Highly Active Doxorubicin Derivative" Nanomaterials 8, no. 2: 110. https://doi.org/10.3390/nano8020110
APA StyleStella, B., Peira, E., Dianzani, C., Gallarate, M., Battaglia, L., Gigliotti, C. L., Boggio, E., Dianzani, U., & Dosio, F. (2018). Development and Characterization of Solid Lipid Nanoparticles Loaded with a Highly Active Doxorubicin Derivative. Nanomaterials, 8(2), 110. https://doi.org/10.3390/nano8020110








