Preparation and Characterization of Antimicrobial Films Based on LDPE/Ag Nanoparticles with Potential Uses in Food and Health Industries
Abstract
:1. Introduction
2. Results
2.1. Film Homogeneity and Nanoparticle Dispersion
2.2. Thermal Characterization
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Thermogravimetric Analysis (TGA)
2.3. Contact Angle Measurements
2.4. Bacterial Cultures
2.4.1. Kirby-Bauer Diffusion Test
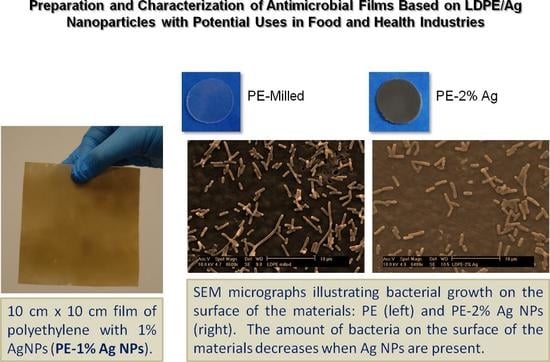
2.4.2. Study of Biofilm Development and Bacterial Growth on the Surface of the Materials
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Characterization Techniques
3.4. Bacterial Cultures
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization. FAO Preventing E. coli in FOOD; Food and Agriculture Organization: Rome, Italy, 2016; pp. 1–15. [Google Scholar]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Barros-Velázquez, J. Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128007235. [Google Scholar]
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Song, X.; Li, R.; Li, H.; Hu, Z.; Mustapha, A.; Lin, M. Characterization and Quantification of Zinc Oxide and Titanium Dioxide Nanoparticles in Foods. Food Bioprocess Technol. 2014, 7, 456–462. [Google Scholar] [CrossRef]
- Arroyo, J.M.; Olmos, D.; Orgaz, B.; Puga, C.H.; San José, C.; González-Benito, J. Effect of the presence of titania nanoparticles in the development of Pseudomonas fluorescens biofilms on LDPE. RSC Adv. 2014, 4, 51451–51458. [Google Scholar] [CrossRef]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surfaces A 2013. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Schadler, L.S. Nanocomposite Science and Technology; Ajayan, P.M., Schadler, L.S., Braun, P.V., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; ISBN 9783527303595. [Google Scholar]
- Hood, M.A.; Mari, M.; Muñoz-Espí, R. Synthetic strategies in the preparation of polymer/inorganic hybrid nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.A.; Torkelson, J.M. Importance of superior dispersion versus filler surface modification in producing robust polymer nanocomposites: The example of polypropylene/nanosilica hybrids. Polymer 2015, 68, 147–157. [Google Scholar] [CrossRef]
- Kim, D.H.; Fasulo, P.D.; Rodgers, W.R.; Paul, D.R. Effect of the ratio of maleated polypropylene to organoclay on the structure and properties of TPO-based nanocomposites. Part II: Thermal expansion behavior. Polymer 2008, 49, 2492–2506. [Google Scholar] [CrossRef]
- Thakur, V.K.; Vennerberg, D.; Kessler, M.R. Green Aqueous Surface Modification of Polypropylene for Novel Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 9349–9356. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, P.D.; Olmos, D.; Amador, D.R.; González-Benito, J. Real dispersion of isolated fumed silica nanoparticles in highly filled PMMA prepared by high energy ball milling. J. Colloid Interface Sci. 2007, 308, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Benito, J.; Martinez-Tarifa, J.; Sepúlveda-García, M.E.; Portillo, R.A.; Gonzalez-Gaitano, G. Composites based on HDPE filled with BaTiO3 submicrometric particles. Morphology, structure and dielectric properties. Polym. Test. 2013, 32, 1342–1349. [Google Scholar] [CrossRef]
- Olmos, D.; Domínguez, C.; Castrillo, P.D.; Gonzalez-Benito, J. Crystallization and final morphology of HDPE: Effect of the high energy ball milling and the presence of TiO2 nanoparticles. Polymer 2009, 50, 1732–1742. [Google Scholar] [CrossRef]
- Olmos, D.; Montero, F.; González-Gaitano, G.; González-Benito, J. Structure and morphology of composites based on polyvinylidene fluoride filled with BaTiO3 submicrometer particles: Effect of processing and filler content. Polym. Compos. 2013, 34, 2094–2104. [Google Scholar] [CrossRef]
- Sanchez, F.A.; González-Benito, J. PVDF/BaTiO3/carbon nanotubes ternary nanocomposites: Effect of nanofillers and processing. Polym. Compos. 2017. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Tortora, M.; Vittoria, V.; Costantino, U.; Marmottini, F.; Padella, F. Incorporation of Mg–Al hydrotalcite into a biodegradable Poly(ε-caprolactone) by high energy ball milling. Polymer 2005, 46, 1601–1608. [Google Scholar] [CrossRef]
- Olmos, D.; García-Lopez, A.; González-Benito, J. Detection in a single experiment of thermal transitions of the constituents in PS/BaTiO3 composites. Mater. Lett. 2013, 97, 8–10. [Google Scholar] [CrossRef]
- Gonzalez-Benito, J. Javier González-Benito High Energy Ball Milling as a New Tool for Preparing Polymer Nanocomposites. In Advances in Nanotechnology; Bartul, Z., Trenor, J., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; Volume 16, pp. 1–26. ISBN 978-1-63485-977-6. [Google Scholar]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Solimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on reducing pasteurization temperature of orange juice. J. Food Process. Preserv. 2012, 36, 104–112. [Google Scholar] [CrossRef]
- Jokar, M.; Abdul Rahman, R.; Ibrahim, N.A.; Abdullah, L.C.; Tan, C.P. Melt Production and Antimicrobial Efficiency of Low-Density Polyethylene (LDPE)-Silver Nanocomposite Film. Food Bioprocess Technol. 2012, 5, 719–728. [Google Scholar] [CrossRef]
- Stará, H.; Staraý, Z.; Münstedt, H. Silver nanoparticles in blends of polyethylene and a superabsorbent polymer: Morphology and silver ion release. Macromol. Mater. Eng. 2011, 296, 423–427. [Google Scholar] [CrossRef]
- Su, Q.Z.; Lin, Q.B.; Chen, C.F.; Wu, L.B.; Wang, Z.W. Effect of organic additives on silver release from nanosilver–polyethylene composite films to acidic food simulant. Food Chem. 2017, 228, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.S.; Webb, P.B.; Tokareva, N.V.; Krasnov, A.P.; Popov, V.K.; Zhang, J.; Roberts, C.J.; Howdle, S.M. Synthesis and characterisation of advanced UHMWPE/silver nanocomposites for biomedical applications. Eur. Polym. J. 2007, 43, 307–314. [Google Scholar] [CrossRef]
- Zapata, P.A.; Tamayo, L.; Páez, M.; Cerda, E.; Azócar, I.; Rabagliati, F.M. Nanocomposites based on polyethylene and nanosilver particles produced by metallocenic “in situ” polymerization: Synthesis, characterization, and antimicrobial behavior. Eur. Polym. J. 2011. [Google Scholar] [CrossRef]
- Zapata, P.A.; Larrea, M.; Tamayo, L.; Rabagliati, F.M.; Azócar, M.I.; Páez, M. Polyethylene/silver-nanofiber composites: A material for antibacterial films. Mater. Sci. Eng. C 2016, 69, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valdes, S.; Ortega-Ortiz, H.; Ramos-de Valle, L.F.; Medellín-Rodríguez, J.F.; Guedea-Miranda, R. Mechanical and Antimicrobial Properties of Multilayer Films with a Polyethylene/Silver Nanocomposite Layer. J. Appl. Polym. Sci. 2009, 111, 953–962. [Google Scholar] [CrossRef]
- Anh, D.H.; Dumri, K.; Anh, N.T.; Punyodom, W.; Rachtanapun, P. Facile fabrication of polyethylene/silver nanoparticle nanocomposites with silver nanoparticles traps and holds early antibacterial effect. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Dehnavi, A.S.; Aroujalian, A.; Raisi, A.; Fazel, S. Preparation and characterization of polyethylene/silver nanocomposite films with antibacterial activity. J. Appl. Polym. Sci. 2013, 127, 1180–1190. [Google Scholar] [CrossRef]
- Sadeghnejad, A.; Aroujalian, A.; Raisi, A.; Fazel, S. Antibacterial nano silver coating on the surface of polyethylene films using corona discharge. Surf. Coat. Technol. 2014, 245, 1–8. [Google Scholar] [CrossRef]
- Jokar, M.; Rahman, R.A.; Abdullah, L.C. Physical and Antimicrobial Characterization of Self Assembled Silver Nanoparticle/Chitosan onto Low Density Polyethylene Film as Active Packaging Polymer. J. Nano Res. 2014, 27, 53–64. [Google Scholar] [CrossRef]
- Olmos, D.; Rodríguez-Gutiérrez, E.; González-Benito, J. Polymer Structure and Morphology of Low Density Polyethylene Filled with Silica Nanoparticles. Polym. Compos. 2012, 33, 2009–2021. [Google Scholar] [CrossRef]
- Men, Y.; Rieger, J.; Endeler, H.F.; Lilge, D. Mechanical α-Process in Polyethylene †. Macromolecules 2003, 36, 4689–4691. [Google Scholar] [CrossRef]
- Mandelkern, L. Crystallization of Polymers in Equilibrium Concepts, 2nd ed.; Cambridge University Press: New York, NY, USA, 2002; Volume 1. [Google Scholar]
- Gonzalez-Benito, J.; González-Gaitano, G.; Olmos, D. Effect of a silica nanofiller on the structure, dynamics and thermostability of LDPE in LDPE/silica nanocomposites. RSC Adv. 2015, 5, 34979–34984. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Saeki, D.; Nagashima, Y.; Sawada, I.; Matsuyama, H. Physicochemical and Engineering Aspects Effect of hydrophobicity of polymer materials used for water purification membranes on biofilm formation dynamics. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 622–628. [Google Scholar] [CrossRef]
- Teno, J.; González-Gaitano, G.; González-Benito, J. Poly (ethylene-co-vinyl acetate) films prepared by solution blow spinning: Surface characterization and its relation with E. coli adhesion. Polym. Test. 2017, 60, 140–148. [Google Scholar] [CrossRef]
- González-Benito, J.; Teno, J.; González-Gaitano, G.; Xu, S.; Chiang, M.Y. PVDF/TiO2 nanocomposites prepared by solution blow spinning: Surface properties and their relation with S. Mutans adhesion. Polym. Test. 2017, 58, 21–30. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Addo Ntim, S.; Thomas, T.A.; Begley, T.H.; Noonan, G.O. Characterisation and potential migration of silver nanoparticles from commercially available polymeric food contact materials. Food Addit. Contam. 2015, 32, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1918; Volume 26, pp. 98–100. [Google Scholar]
- Longford, J.I.L.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]










| Sample | Tm,1 (°C) (1st Scan) | Tm,2 (°C) (2nd Scan) | Tc (°C) (Cooling) | Xc |
|---|---|---|---|---|
| PE-Ground | 112.6 | 109.8 | 96.7 | 0.30 |
| PE-Milled | 111.3 | 109.7 | 98.5 | 0.29 |
| PE-0.5% Ag | 110.0 | 109.7 | 98.5 | 0.29 |
| PE-1% Ag | 110.7 | 110.2 | 98.4 | 0.30 |
| PE-2% Ag | 111.3 | 110.5 | 98.1 | 0.29 |
| Sample | T5 (°C) | T50 (°C) | T95 (°C) | Tpeak,DTGA (°C) | Residue (Mass %) |
|---|---|---|---|---|---|
| PE-Ground | 403 ± 2 | 456 ± 2 | 481 ± 1 | 466 ± 0 | 0.0 |
| PE-Milled | 395 ± 6 | 456 ± 1 | 484 ± 1 | 465 ± 0 | 0.0 |
| PE-0.5% Ag | 408 ± 6 | 458 ± 4 | 481 ± 2 | 467 ± 3 | 0.7 |
| PE-1% Ag | 406 ± 2 | 459 ± 2 | 483 ± 2 | 466 ± 1 | 1.9 |
| PE-2% Ag | 410 ± 8 | 463 ± 1 | 486 ± 1 | 470 ± 3 | 2.2 |
| Sample | Water | Glycerol | Diiodomethane |
|---|---|---|---|
| PE-Ground | 101 ± 2 | 95 ± 2 | 74 ± 1 |
| PE-Milled | 95 ± 2 | 94 ± 1 | 73 ± 2 |
| PE-0.5% Ag | 93 ± 2 | 96 ± 1 | 72 ± 3 |
| PE-1% Ag | 94 ± 1 | 93 ± 2 | 73 ± 2 |
| PE-2% Ag | 97 ± 1 | 95 ± 1 | 72 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmos, D.; Pontes-Quero, G.M.; Corral, A.; González-Gaitano, G.; González-Benito, J. Preparation and Characterization of Antimicrobial Films Based on LDPE/Ag Nanoparticles with Potential Uses in Food and Health Industries. Nanomaterials 2018, 8, 60. https://doi.org/10.3390/nano8020060
Olmos D, Pontes-Quero GM, Corral A, González-Gaitano G, González-Benito J. Preparation and Characterization of Antimicrobial Films Based on LDPE/Ag Nanoparticles with Potential Uses in Food and Health Industries. Nanomaterials. 2018; 8(2):60. https://doi.org/10.3390/nano8020060
Chicago/Turabian StyleOlmos, Dania, Gloria María Pontes-Quero, Angélica Corral, Gustavo González-Gaitano, and Javier González-Benito. 2018. "Preparation and Characterization of Antimicrobial Films Based on LDPE/Ag Nanoparticles with Potential Uses in Food and Health Industries" Nanomaterials 8, no. 2: 60. https://doi.org/10.3390/nano8020060
APA StyleOlmos, D., Pontes-Quero, G. M., Corral, A., González-Gaitano, G., & González-Benito, J. (2018). Preparation and Characterization of Antimicrobial Films Based on LDPE/Ag Nanoparticles with Potential Uses in Food and Health Industries. Nanomaterials, 8(2), 60. https://doi.org/10.3390/nano8020060