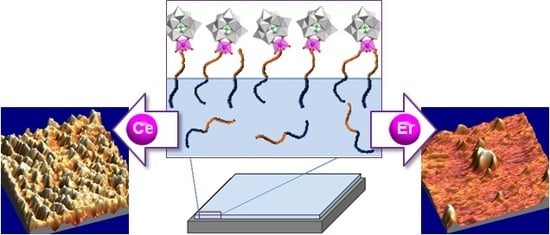
Immobilization of Polyoxometalates on Tailored Polymeric Surfaces
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building Blocks for Functional Nanoscale Systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid Organic–Inorganic Polyoxometalate Compounds: From Structural Diversity to Applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, Germany, 1983. [Google Scholar]
- Pope, M.T.; Müller, A. (Eds.) Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity; Kluwer: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Pope, M.T.; Müller, A. (Eds.) Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications; Kluwer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Pope, M.T.; Yamase, T. (Eds.) Polyoxometalate Chemistry for Nanocomposite Design; Kluwer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Borrás-Almenar, J.J.; Coronado, E.; Müller, A.; Pope, M.T. (Eds.) Polyoxometalate Molecular Science; Kluwer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Sécheresse, F. (Ed.) Polyoxometalate Chemistry. Some Recent Trends; World Scientific: Singapore, 2013. [Google Scholar]
- Hill, C.L. Introduction: PolyoxometalatesMulticomponent Molecular Vehicles to Probe Fundamental Issues and Practical Problems. Chem. Rev. 1998, 98, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kortz, U. Polyoxometalates. Eur. J. Inorg. Chem. 2009, 2009, 5056. [Google Scholar] [CrossRef]
- Cronin, L.; Long, D.-L.; Rosnes, M.H.; Yvon, C. Mapping the synthesis of low nuclearity polyoxometalates from octamolybdates to Mn-Anderson clusters. Dalton Trans. 2012, 41, 10071–10079. [Google Scholar]
- Cronin, L.; Müller, A. From serendipity to design of polyoxometalates at the nanoscale, aesthetic beauty and applications. Chem. Soc. Rev. 2012, 41, 7333–7334. [Google Scholar] [CrossRef] [PubMed]
- Kortz, U.; Liu, T. The Best of Polyoxometalates. Eur. J. Inorg. Chem. 2013, 2013, 1559–1560. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tang, Z.Y. Polyoxometalate-based functional nanostructured films: Current progress and future prospects. Nano Today 2010, 5, 267–281. [Google Scholar] [CrossRef]
- Li, D.; Yin, P.; Liu, T. Supramolecular architectures assembled from amphiphilic hybrid polyoxometalates. Dalton Trans. 2012, 41, 2853–2861. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.; Lu, Y.; Zhuang, J.; Wang, X. Cluster-Based Self-Assembly: Reversible Formation of Polyoxometalate Nanocones and Nanotubes. Chem. Mater. 2009, 21, 3745–3751. [Google Scholar] [CrossRef]
- Kurth, D.G.; Lehmann, P.; Volkmer, D.; Cölfen, H.; Müller, A.; Du Chesne, A. Surfactant-Encapsulated Clusters (SECs): (DODA)20(NH4)[H3Mo57V6(NO)6O183(H2O)18], a Case Study. Chem. Eur. J. 2000, 6, 385–393. [Google Scholar] [CrossRef]
- Li, H.; Sun, H.; Qi, H.; Xu, M.; Wu, L. Onionlike Hybrid Assemblies Based on Surfactant-Encapsulated Polyoxometalates. Angew. Chem. Int. Ed. 2007, 46, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Landsmann, S.; Lizandara-Pueyo, C.; Polarz, S. Bolaform surfactants with polyoxometalate head groups and their assembly into ultra-small monolayer membrane vesicles. J. Am. Chem. Soc. 2010, 132, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Y.F.; Cronin, L.; Liu, T. Self-assembly of organic-inorganic hybrid amphiphilic surfactants with large polyoxometalates as polar head groups. J. Am. Chem. Soc. 2008, 130, 14408–14409. [Google Scholar] [CrossRef] [PubMed]
- Rosnes, M.H.; Musumeci, C.; Pradeep, C.P.; Mathieson, J.S.; Long, D.-L.; Song, Y.-F.; Pignataro, B.; Cogdell, R.; Cronin, L. Assembly of Modular Asymmetric Organic-Inorganic Polyoxometalate Hybrids into Anisotropic Nanostructures. J. Am. Chem. Soc. 2010, 132, 15490–15492. [Google Scholar] [CrossRef] [PubMed]
- Toma, F.M.; Sartorel, A.; Iurlo, M.; Carraro, M.; Parisse, P.; Maccato, C.; Rapino, S.; Rodriguez Gonzalez, B.; Amenitsch, H.; Da Ros, T.; et al. Efficient water oxidation at carbon nanotube–polyoxometalate electrocatalytic interfaces. Nat. Chem. 2010, 2, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Giusti, A.; Charron, G.; Mazerat, S.; Compain, J.-D.; Mialane, P.; Dolbecq, A.; Rivière, E.; Wernsdorfer, W.; Ngo Biboum, R.; Keita, B.; et al. Magnetic Bistability of Individual Single-Molecule Magnets Grafted on Single-Wall Carbon Nanotubes. Angew. Chem. Int. Ed. 2009, 48, 4949–4952. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.-P.; Goubert-Renaudin, S.; Alia, S.; Yan, Y.; Barteau, M.A. Structure, Stability, and Electronic Interactions of Polyoxometalates on Functionalized Graphene Sheets. Langmuir 2013, 29, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pang, S.; Wu, S.; Feng, X.; Müllen, K.; Bubeck, C. Layer-by-Layer Assembly and UV Photoreduction of Graphene–Polyoxometalate Composite Films for Electronics. J. Am. Chem. Soc. 2011, 133, 9423–9429. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Luo, Z.; Britt, D.K.; Furukawa, H.; Yaghi, O.M.; Hardcastle, K.I.; Hill, C.L. A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate Metal Organic Framework for Aerobic Decontamination. J. Am. Chem. Soc. 2011, 133, 16839–16846. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.R.; Thouvenot, R.; Lalot, T. New Hybrid Covalent Networks Based on Polyoxometalates: Part 1. Hybrid Networks Based on Poly(ethyl methacrylate) Chains Covalently Cross-linked by Heteropolyanions: Synthesis and Swelling Properties. Chem. Mater. 2000, 12, 257–260. [Google Scholar]
- Han, J.W.; Hill, C.L. A coordination network that catalyzes O2-based oxidations. J. Am. Chem. Soc. 2007, 129, 15094–15095. [Google Scholar] [CrossRef] [PubMed]
- Geisberger, G.; Paulus, S.; Carraro, M.; Bonchio, M.; Patzke, G.R. Synthesis, Characterisation and Cytotoxicity of Polyoxometalate/Carboxymethyl Chitosan Nanocomposites. Chem. Eur. J. 2011, 17, 4619–4625. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Uchida, S.; Mizuno, N. Micelles and Vesicles Formed by Polyoxometalate–Block Copolymer Composites. Angew. Chem. Int. Ed. 2009, 48, 8281–8284. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, W.; Li, W.; Sun, H.; Bu, W.; Wu, L. A Highly Transparent and Luminescent Hybrid Based on the Copolymerization of Surfactant-Encapsulated Polyoxometalate and Methyl Methacrylate. Adv. Mater. 2005, 17, 2688–2692. [Google Scholar] [CrossRef]
- Ammam, M. Polyoxometalates: Formation, structures, principal properties, main deposition methods and application in sensing. J. Mater. Chem. A 2013, 1, 6291–6312. [Google Scholar] [CrossRef]
- Carraro, M.; Gardan, M.; Scorrano, G.; Drioli, E.; Fontananova, E.; Bonchio, M. Solvent-free, heterogeneous photooxygenation of hydrocarbons by Hyflon® membranes embedding a fluorous-tagged decatungstate. Chem. Commun. 2006, 4533–4535. [Google Scholar] [CrossRef]
- Fontananova, E.; Donato, L.; Drioli, E.; Lopez, L.C.; Favia, P.; d’Agostino, R. Heterogenization of Polyoxometalates on the Surface of Plasma-Modified Polymeric Membranes. Chem. Mater. 2006, 18, 1561–1568. [Google Scholar] [CrossRef]
- Alam, M.S.; Dremov, V.; Müller, P.; Postnikov, A.V.; Mal, S.S.; Hussain, F.; Kortz, U. STM/STS Observation of Polyoxoanions on HOPG Surfaces: The Wheel-Shaped [Cu20Cl(OH)24(H2O)12(P8W48O184)]25− and the Ball-Shaped [{Sn(CH3)2(H2O)}24{Sn(CH3)2}12(A-PW9O34)12]36−. Inorg. Chem. 2006, 45, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, C.; Luzio, A.; Pradeep, C.P.; Miras, H.N.; Rosnes, M.H.; Song, Y.-F.; Long, D.-L.; Cronin, L.; Pignataro, B. Programmable Surface Architectures Derived from Hybrid Polyoxometalate-Based Clusters. J. Phys. Chem. C 2011, 115, 4446–4455. [Google Scholar] [CrossRef]
- Inumaru, K.; Ishihara, T.; Kamiya, Y.; Okuhara, T.; Yamanaka, S. Water-Tolerant, Highly Active Solid Acid Catalysts Composed of the Keggin-Type Polyoxometalate H3PW12O40 Immobilized in Hydrophobic Nanospaces of Organomodified Mesoporous Silica. Angew. Chem. Int. Ed. 2007, 46, 7625–7628. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Perkas, N.; Li, Q.-L.; Gofer, Y.; Koltypin, Y.; Gedanken, A. Improved Silanization Modification of a Silica Surface and Its Application to the Preparation of a Silica-Supported Polyoxometalate Catalyst. Langmuir 2003, 19, 10409–10413. [Google Scholar] [CrossRef]
- Kasai, J.; Nakagawa, Y.; Uchida, S.; Yamaguchi, K.; Mizuno, N. [γ-1,2-H2SiV2W10O40] Immobilized on Surface-Modified SiO2 as a Heterogeneous Catalyst for Liquid-Phase Oxidation with H2O2. Chem. Eur. J. 2006, 12, 4176–4184. [Google Scholar] [CrossRef] [PubMed]
- Song, I.K.; Kaba, M.S.; Nomiya, K.; Finke, R.G.; Barteau, M.A. Scanning tunneling microscopy (STM) and tunneling spectroscopy (TS) studies of polyoxometalates (POMs) of the Wells–Dawson structural class. J. Mol. Catal. A 2007, 262, 216–226. [Google Scholar] [CrossRef]
- Zhong, D.; Sousa, F.L.; Müller, A.; Chi, L.; Fuchs, H. A Nanosized Molybdenum Oxide Wheel with a Unique Electronic-Necklace Structure: STM Study with Submolecular Resolution. Angew. Chem. Int. Ed. 2011, 50, 7018–7021. [Google Scholar] [CrossRef] [PubMed]
- Errington, R.J.; Petkar, S.S.; Horrocks, B.R.; Houlton, A.; Lie, L.H.; Patole, S.N. Covalent Immobilization of a TiW5 Polyoxometalate on Derivatized Silicon Surfaces. Angew. Chem. Int. Ed. 2005, 44, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Schroden, R.C.; Blanford, C.F.; Melde, B.J.; Johnson, B.J.S.; Stein, A. Direct Synthesis of Ordered Macroporous Silica Materials Functionalized with Polyoxometalate Clusters. Chem. Mater. 2001, 13, 1074–1081. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, C. A novel polyoxometalate-functionalized mesoporous hybrid silica: Synthesis and characterization. J. Mater. Chem. 2008, 18, 2691–2703. [Google Scholar] [CrossRef]
- Mercier, D.; Boujday, S.; Annabi, C.; Villanneau, R.; Pradier, C.-M.; Proust, A. Bifunctional Polyoxometalates for Planar Gold Surface Nanostructuration and Protein Immobilization. J. Phys. Chem. C 2012, 116, 13217–13224. [Google Scholar] [CrossRef]
- Villanneau, R.; Marzouk, A.; Wang, Y.; Djamaa, A.B.; Laugel, G.; Proust, A.; Launay, F. Covalent Grafting of Organic–Inorganic Polyoxometalates Hybrids onto Mesoporous SBA-15: A Key Step for New Anchored Homogeneous Catalysts. Inorg. Chem. 2013, 52, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; McMillan, N.; Long, D.-L.; Kane, S.; Malm, J.; Riehle, M.O.; Pradeep, C.P.; Gadegaard, N.; Cronin, L. Micropatterned Surfaces with Covalently Grafted Unsymmetrical Polyoxometalate-Hybrid Clusters Lead to Selective Cell Adhesion. J. Am. Chem. Soc. 2009, 131, 1340–1341. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.S.; Stein, A. Surface Modification of Mesoporous, Macroporous, and Amorphous Silica with Catalytically Active Polyoxometalate Clusters. Inorg. Chem. 2001, 40, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y.; Hu, C.; Wang, Y.; Wang, E. Preparation of surface modifications of mesoporous titania with monosubstituted Keggin units and their catalytic performance for organochlorine pesticide and dyes under UV irradiation. Appl. Catal. A 2004, 273, 201–210. [Google Scholar] [CrossRef]
- Bousquet, A.; Ibarboure, E.; Teran, F.J.; Ruiz, L.; Garay, M.T.; Laza, J.M.; Vilas, J.L.; Papon, E.; Rodríguez-Hernández, J. pH responsive surfaces with nanoscale topography. J. Polym. Sci. A Polym. Chem. 2010, 48, 2982–2990. [Google Scholar] [CrossRef]
- Ruiz, L.; Garay, M.T.; Laza, J.M.; Vilas, J.L.; Rodríguez-Hernández, J.; Labrugere, C.; León, L.M. Reversible functionalization of nanostructured polymer surfaces via stimuli-responsive interpolymer complexes. Eur. Polym. J. 2013, 49, 130–138. [Google Scholar] [CrossRef]
- Sadakane, M.; Dickman, M.H.; Pope, M.T. Controlled Assembly of Polyoxometalate Chains from Lacunary Building Blocks and Lanthanide-Cation Linkers. Angew. Chem. Int. Ed. 2000, 39, 2914–2916. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, P.; Chen, H.; Wang, J.; Niu, J. Synthesis, structure, and properties of a 1-D cerium based on monovacant Keggin-type polyoxotungstate. J. Coord. Chem. 2011, 64, 2178–2185. [Google Scholar] [CrossRef]
- Mialane, P.; Lisnard, L.; Mallard, A.; Marrot, J.; Antic-Fidancev, E.; Aschehoug, P.; Vivien, D.; Sécheresse, F. Solid-State and Solution Studies of {Lnn(SiW11O39)} Polyoxoanions: An Example of Building Block Condensation Dependent on the Nature of the Rare Earth. Inorg. Chem. 2003, 42, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhang, S.-X.; Li, S.-J.; Gao, Y.-H.; Zhang, X.; Wang, X.-N.; Liu, S.-X. pH-Controlled assembly of two polyoxometalate chains based on [α-GeW11O39]8− and Eu3+: Syntheses, crystal structures, and properties. J. Coord. Chem. 2011, 64, 4006–4015. [Google Scholar] [CrossRef]
- Wang, J.-P.; Yan, Q.-X.; Du, X.-D.; Duan, X.-Y.; Niu, J.-Y. Synthesis, crystal structures and properties of three rare earth substituted germanotungstates: M/[α-GeW11O39] (M = Nd, Eu, and Tb). Inorg. Chim. Acta 2008, 361, 2701–2706. [Google Scholar] [CrossRef]
- Chakraborty, S.; Jin, L.; Li, Y.; Liu, Y.; Dutta, T.; Zhu, D.-M.; Yan, X.; Keightley, A.; Peng, Z. Synthesis, Characterizations, and Morphological Studies of Polyoxometalate-Containing Rod–Coil Diblock Copolymers. Eur. J. Inorg. Chem. 2013, 1799–1807. [Google Scholar] [CrossRef]
- Li, Y.; Jin, L.; Chakraborty, S.; Li, S.; Lu, P.; Zhu, D.-M.; Yan, X.; Peng, Z. Photovoltaic properties and femtosecond time-resolved fluorescence study of polyoxometalate-containing rod–coil diblock copolymers. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 122–133. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Shetye, K.; Dutta, T.; Jin, L.; Li, S.; Peng, Z. Luminescent Polythiophene-Based Main-Chain Polyoxometalate-Containing Conjugated Polymers with Improved Solar-Cell Performance. Eur. J. Inorg. Chem. 2015, 656–663. [Google Scholar] [CrossRef]
- Lunkenbein, T.; Kamperman, M.; Li, Z.; Bojer, C.; Drechsler, M.; Förster, S.; Wiesner, U.; Müller, A.H.E.; Breu, J. Direct synthesis of inverse hexagonally ordered diblock copolymer/polyoxometalate nanocomposite films. J. Am. Chem. Soc. 2012, 134, 12685–12692. [Google Scholar] [CrossRef] [PubMed]
- Tézé, A.; Hervé, G.; Finke, R.G.; Lyon, D.K. Inorganic Syntheses; Ginsberg, P.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1990; Volume 27, pp. 85–96. [Google Scholar]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.C.; Órfao, J.J.M.; Pereira, M.F.R. A novel ceria–activated carbon composite for the catalytic ozonation of carboxylic acids. Catal. Commun. 2008, 9, 2121–2126. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Li, K.; Chai, F.; Zhao, Y.; Guo, X. Facile synthesis of magnetic Fe3O4/CeCO3OH composites with excellent adsorption capability for small cationic dyes. RSC Adv. 2015, 5, 94397–94404. [Google Scholar] [CrossRef]






| Element | Peak and BE (eV) | Atom (%) | ||
|---|---|---|---|---|
| Ce | OPAA-Ce-OPOM | Ce 3d5/2 883.0 | Ce 3d3/2 901.3 | 0.18 |
| Ce-OPAA | Ce 3d5/2 886.7 | Ce 3d3/2 905.2 | 0.25 | |
| Total = 0.40 | ||||
| W | W4f | 35.6 | ||
| Total = 1.9 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado-Ureta, S.; Rodríguez-Hernández, J.; Del Campo, A.; Perez-Álvarez, L.; Ruiz-Rubio, L.; Vilas, J.L.; Artetxe, B.; Reinoso, S.; Gutiérrez-Zorrilla, J.M. Immobilization of Polyoxometalates on Tailored Polymeric Surfaces. Nanomaterials 2018, 8, 142. https://doi.org/10.3390/nano8030142
Aguado-Ureta S, Rodríguez-Hernández J, Del Campo A, Perez-Álvarez L, Ruiz-Rubio L, Vilas JL, Artetxe B, Reinoso S, Gutiérrez-Zorrilla JM. Immobilization of Polyoxometalates on Tailored Polymeric Surfaces. Nanomaterials. 2018; 8(3):142. https://doi.org/10.3390/nano8030142
Chicago/Turabian StyleAguado-Ureta, Saioa, Juan Rodríguez-Hernández, Adolfo Del Campo, Leyre Perez-Álvarez, Leire Ruiz-Rubio, José Luis Vilas, Beñat Artetxe, Santiago Reinoso, and Juan M. Gutiérrez-Zorrilla. 2018. "Immobilization of Polyoxometalates on Tailored Polymeric Surfaces" Nanomaterials 8, no. 3: 142. https://doi.org/10.3390/nano8030142
APA StyleAguado-Ureta, S., Rodríguez-Hernández, J., Del Campo, A., Perez-Álvarez, L., Ruiz-Rubio, L., Vilas, J. L., Artetxe, B., Reinoso, S., & Gutiérrez-Zorrilla, J. M. (2018). Immobilization of Polyoxometalates on Tailored Polymeric Surfaces. Nanomaterials, 8(3), 142. https://doi.org/10.3390/nano8030142