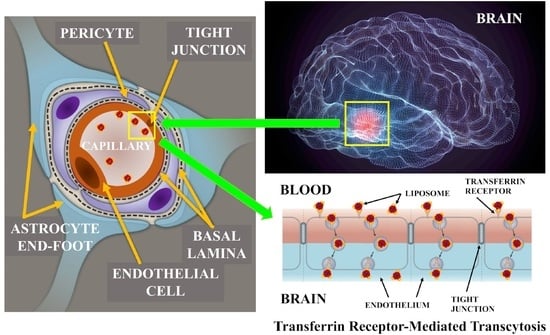
Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood–Brain Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantification of DA∙HCl
2.3. Preparation of Unfunctionalized LPs
2.4. Preparation of Tf Functionalized LPs
2.5. Physicochemical Characterization of LPs
2.6. Quantification of DA∙HCl into LPs
2.7. Freeze-Fracture Electron Microscopy
2.8. In Vitro Release Studies
2.9. Stability Studies
2.10. Culture of hCMEC/D3 Cells and Endothelial Permeability Experiments
2.11. Statistical Analysis
3. Results and Discussion
3.1. LPs Characterization
3.2. In Vitro Release Studies
3.3. In Vitro Transport Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spuch, C.; Navarro, C. Liposomes for targeted delivery of active agents against neurodegenerative diseases (Alzheimer’s disease and Parkinson’s Disease). J. Drug Deliv. 2011, 2011, 469679. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Ali, H.; Denora, N.; Rytting, E. Oxcarbazepine-loaded polymeric nanoparticles: Development and permeability studies across in vitro models of the blood-brain barrier and human placental trophoblast. Int. J. Nanomed. 2015, 10, 1985–1996. [Google Scholar]
- Bjorklund, A.; Dunnett, S.B. Dopamine neuron system in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Cassano, T.; Laquintana, V.; Lopalco, A.; Trapani, A.; Cimmino, C.S.; Laconca, L.; Giuffrida, A.; Trapani, G. Novel codrugs with GABAergic activity for dopamine delivery in the brain. Int. J. Pharm. 2012, 437, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Cassano, T.; Lopalco, A.; de Candia, M.; Laquintana, V.; Lopedota, A.; Cutrignelli, A.; Perrone, M.; Iacobazzi, R.M.; Bedse, G.; Franco, M.; et al. Oxazepam-Dopamine Conjugates Increase Dopamine Delivery into Striatum of Intact Rats. Mol. Pharm. 2017, 14, 3178–3187. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Sozio, P.; Iannitelli, A.; Cerasa, L.S. New drug delivery strategies for improved Parkinson’s disease therapy. Expert Opin. Drug Deliv. 2009, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Denora, N.; Cutrignelli, A.; Perrone, M.; Iacobazzi, R.; Annese, C.; Lopalco, A.; Lopedota, A.; Franco, M. TSPO ligand-methotrexate prodrug conjugates: Design, synthesis, and biological evaluation. Int. J. Mol. Sci. 2016, 17, 967. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, R.M.; Lopalco, A.; Cutrignelli, A.; Laquintana, V.; Lopedota, A.; Franco, M.; Denora, N. Bridging Pharmaceutical Chemistry with Drug and Nanoparticle Targeting to Investigate the Role of the 18-kDa Translocator Protein TSPO. ChemMedChem 2017, 12, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Beesu, M.; Caruso, G.; Salyer, A.C.; Shukla, N.M.; Khetani, K.K.; Smith, L.J.; Fox, L.M.; Tanji, H.; Ohto, U.; Shimizu, T.; et al. Identification of a Human Toll-Like Receptor (TLR) 8-Specific Agonist and a Functional Pan-TLR Inhibitor in 2-Aminoimidazoles. J. Med. Chem. 2016, 59, 3311–3330. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Lopalco, A.; Iacobazzi, R.M.; Lopedota, A.; Cutrignelli, A.; Iacobellis, G.; Annese, C.; Cascione, M.; Leporatti, S.; et al. In vitro targeting and imaging the translocator protein TSPO. J. Control. Release 2013, 172, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Denora, N.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Lasorsa, F.M.; Agostino, G.; Franco, M. Translocator protein ligand-PLGA conjugated nanoparticles for 5-fluorouracil delivery to glioma cancer cells. Mol. Pharm. 2014, 11, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Annese, C.; Abbrescia, D.I.; Catucci, L.; D’Accolti, L.; Denora, N.; Fanizza, I.; Fusco, C.; La Piana, G. Site-dependent biological activity of valinomycin analogs bearing derivatizable hydroxyl sites. J. Pept. Sci. 2013, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Warren Olanow, C. Levodopa/dopamine replacement strategies in Parkinson’s disease: Future directions. Mov. Disord. 2008, 23, S613–S622. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Woodward, W.R. Levodopa pharmacokinetics and pharmacodynamics in fluctuating parkinsonian patients. Neurology 1986, 36, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Miyazaki, I.; Ogawa, N. Dopamine- or L-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox. Res. 2003, 5, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Mohanakumar, K.P. L-DOPA induced-endogenous 6-hydroxydopamine is the cause of aggravated dopaminergic neurodegeneration in Parkinson’s disease patients. Med. Hypothesis 2012, 79, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Douglas, J.; Denora, N.; Stella, V. Determination of pKa and hydration constants for a series of α-keto-carboxylic acids using nuclear magnetic resonance spectrometry. J. Pharm. Sci. 2016, 105, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Leto, I.; Coronnello, M.; Righeschi, C.; Bergonzi, M.C.; Mini, E.; Bilia, A.R. Enhanced efficacy of artemisinin loaded in transferrin-conjugated liposomes versus stealth liposomes against HCT-8 colon cancer cells. ChemMedChem 2016, 11, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Sonali; Singh, R.P.; Singh, N.; Sharma, G.; Vijayakumar, M.R.; Koch, B.; Singh, S.; Singh, U.; Dash, D.; Pandey, B.L.; et al. Transferrin liposomes of docetaxel for brain-targeted cancer applications: Formulation and brain theranostics. Drug Deliv. 2016, 23, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Librelotto, D.R.; Codevilla, C.F.; Farooqi, A.; Rolim, C.M.B. Transferrin-Conjugated Nanocarriers as Active-Targeted Drug Delivery Platforms for Cancer Therapy. Curr. Pharm. Des. 2017, 23, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, H.; Jia, X.; Lu, W.L.; Lou, J.; Wei, Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef] [PubMed]
- Lopedota, A.; Trapani, A.; Cutrignelli, A.; Laquintana, V.; Denora, N.; Franco, M.; Trapani, G.; Liso, G. Effect of CDs on physico-chemical and release properties of Eudragit RS 100 microparticles containing glutathione. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 425–432. [Google Scholar] [CrossRef]
- Cutrignelli, A.; Lopedota, A.; Denora, N.; Laquintana, V.; Tongiani, S.; Franco, M. Characterization and release studies of liposomal gels containing glutathione/cyclodextrins complexes potentially useful for cutaneous administration. J. Pharm. Sci. 2014, 103, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Pasko, E.; Vaz Gisela, M.F.; Ehrhardt, C.; Senge, M.O. Trasferrin conjugation does not increase the efficiency of liposomal Foscan during in vitro photodynamic therapy of oesophageal cancer. Eur. J. Pharm. Sci. 2013, 48, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Lopedota, A.; Perrone, M.; Laquintana, V.; Iacobazzi, R.M.; Milella, A.; Fanizza, E.; Depalo, N.; Cutrignelli, A.; Lopalco, A.; et al. Spray-dried mucoadhesives for intravesical drug delivery using N-acetylcysteine- and glutathione-glycol chitosan conjugates. Acta Biomater. 2016, 43, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Denora, N.; Iacobazzi, R.M.; Perrone, M.; Fanizza, E.; Mastrodonato, M.; Mentino, D.; Lopalco, A.; et al. Spray dried chitosan microparticles for intravesical delivery of celecoxib: Preparation and characterization. Pharm. Res. 2016, 33, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- De Campos, R.P.; Siegel, J.M.; Fresta, C.G.; Caruso, G.; Fracassi da Silva, J.A.; Lunte, S.M. Indirect detection of superoxide in RAW 264.7 macrophage cells using microchip electrophoresis coupled to laser induced fluorescence detection. Anal. Bioanal. Chem. 2015, 407, 7003–7012. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.; Gregoriadis, G. Dehydration-Rehydration vesicles: A simple method for high yield drug entrapment in liposomes. Nat. Biotechnol. 1984, 2, 979–984. [Google Scholar] [CrossRef]
- Anabousi, S.; Bakowsky, U.; Schneider, M.; Huwer, H.; Lehr, C.M.; Ehrhardt, C. In vitro assessment of transferrin-conjugated liposomes as drug delivery systems for inhalation therapy of lung cancer. Eur. J. Pharm. Sci. 2006, 29, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Kaur, R.; Henriksen-Lacey, M.; McNeil, S.E.; Wilkhu, J.; Lattmann, E.; Christensen, D.; Mohammed, A.R.; Perrie, Y. Microscopy imaging of liposomes: From coverslips to environmental SEM. Int. J. Pharm. 2011, 417, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Erazo-Oliveras, A.; Muthukrishnan, N.; Baker, R.; Wang, T.Y.; Pellois, J.P. Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals 2012, 5, 1177–1209. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hu, C.M.J.; Fang, R.H.; Zhang, L. Liposome-like nanostructures for drug delivery. J. Mater. Chem. B 2013, 1, 6569–6589. [Google Scholar] [CrossRef] [PubMed]




| Formulation | Size (nm) | PDI | Zeta Potential (mV) | (EE%) |
|---|---|---|---|---|
| unfunctionalized DA∙HCl-LPs | 162.4 ± 3.2 | 0.20 | +4.8 ± 0.9 | 41.5 ± 2.9 |
| Tf functionalized DA∙HCl-LPs | 181.7 ± 7.8 | 0.20 | +7.5 ± 1.2 | 35.4 ± 1.8 |
| Formulation | Week | 1 | Week | 2 | Week | 3 | Week | 4 |
|---|---|---|---|---|---|---|---|---|
| Size (nm) | PDI | Size (nm) | PDI | Size (nm) | PDI | Size (nm) | PDI | |
| unfunctionalized DA∙HCl-LPs | 168.4 ± 2.4 | 0.20 | 165.4 ± 1.8 | 0.25 | 159.4 ± 3.5 | 0.19 | 160.7 ± 1.2 | 0.21 |
| Tf functionalized DA∙HCl-LPs | 186.5 ± 7.8 | 0.20 | 175.7 ± 1.3 | 0.18 | 182.4 ± 4.1 | 0.23 | 179.4 ± 0.8 | 0.18 |
| Formulation | Pe ± SD (cm/min) |
|---|---|
| Unfunctionalized DA·HCl-LPs | 0.92 ± 0.24 × 10−3 |
| Tf Functionalized DA·HCl-LPs | 4.97 ± 0.41 × 10−3 |
| Luciferin yellow | 1.12 ± 0.18 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopalco, A.; Cutrignelli, A.; Denora, N.; Lopedota, A.; Franco, M.; Laquintana, V. Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood–Brain Barrier. Nanomaterials 2018, 8, 178. https://doi.org/10.3390/nano8030178
Lopalco A, Cutrignelli A, Denora N, Lopedota A, Franco M, Laquintana V. Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood–Brain Barrier. Nanomaterials. 2018; 8(3):178. https://doi.org/10.3390/nano8030178
Chicago/Turabian StyleLopalco, Antonio, Annalisa Cutrignelli, Nunzio Denora, Angela Lopedota, Massimo Franco, and Valentino Laquintana. 2018. "Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood–Brain Barrier" Nanomaterials 8, no. 3: 178. https://doi.org/10.3390/nano8030178
APA StyleLopalco, A., Cutrignelli, A., Denora, N., Lopedota, A., Franco, M., & Laquintana, V. (2018). Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood–Brain Barrier. Nanomaterials, 8(3), 178. https://doi.org/10.3390/nano8030178