Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
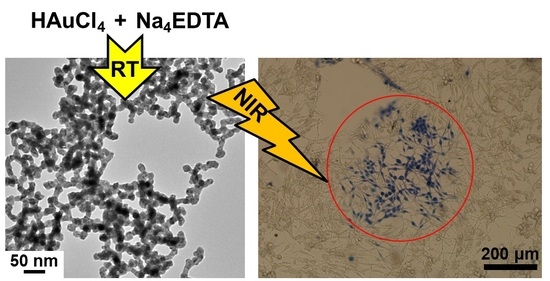
2.2. Shape-Controlled Synthesis of Au Nanostructures
2.3. In Vitro Cytotoxicity against U87MG Cells
2.4. In Vitro Photothermal Therapy
2.5. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmid, G.; Corain, B. Nanoparticulated gold: Syntheses, structures, electronics, and reactivities. Eur. J. Inorg. Chem. 2003, 2003, 3081–3098. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.J.; Brust, M.; Schmidbaur, H. Gold--an introductory perspective. Chem. Soc. Rev. 2008, 37, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chu, W.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.J.; Wang, X.; Yang, W. New gold nanostructures for sensor applications: A review. Materials 2014, 7, 5169–5201. [Google Scholar] [CrossRef] [PubMed]
- Salunke, B.K.; Sathiyamoorthi, E.; Tran, T.K.; Kim, B.S. Phyto-synthesized silver nanoparticles for biological applications. Korean J. Chem. Eng. 2017, 34, 943–951. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 0, 801–802. [Google Scholar] [CrossRef]
- Kim, S.; Jang, Y.; Yoon, K.Y.; Park, J. Surface engineered gold nanoparticles through highly stable metal-surfactant complexes. J. Colloid Interface Sci. 2016, 464, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, S.-H.; Lim, B.; Park, B.J.; Bhang, S.H.; Yu, T. Aqueous-phase synthesis of metal nanoparticles using phosphates as stabilizers. Korean J. Chem. Eng. 2017, 34, 231–233. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Murphy, C.J.; Jana, N.R. Controlling the aspect ratio of inorganic nanorods and nanowires. Adv. Mater. 2002, 14, 80–82. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv. Mater. 2001, 13, 1389–1393. [Google Scholar] [CrossRef]
- Kim, F.; Song, J.H.; Yang, P. Photochemical synthesis of gold nanorods. J. Am. Chem. Soc. 2002, 124, 14316–14317. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Chang, H.-H.; Murphy, C.J. Mini gold nanorods with tunable plasmonic peaks beyond 1000 nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, K.; Lu, Z.; Li, G.; Chen, J.; Deng, Y.; Li, S.; Zhou, F.; He, N. Efficient and facile synthesis of gold nanorods with finely tunable plasmonic peaks from visible to near-IR range. Chem. Mater. 2014, 26, 1794–1798. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Villarreal, E.; Lin, Y.; Zou, S.; Wang, H. Faceted gold nanorods: Nanocuboids, convex nanocuboids, and concave nanocuboids. Nano Lett. 2015, 15, 4161–4169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Wei, W.T.; Yang, C.W.; Huang, M.H. Seed-mediated growth of ultralong gold nanorods and nanowires with a wide range of length tunability. Langmuir 2013, 29, 10491–10497. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yavuz, M.S.; Tuan, H.Y.; Korgel, B.A.; Xia, Y. Ultrathin gold nanowires can be obtained by reducing polymeric strands of oleylamine-aucl complexes formed via aurophilic interaction. J. Am. Chem. Soc. 2008, 130, 8900–8901. [Google Scholar] [CrossRef] [PubMed]
- Pong, B.-K.; Elim, H.I.; Chong, J.-X.; Ji, W.; Trout, B.L.; Lee, J.-Y. New insights on the nanoparticle growth mechanism in the citrate reduction of gold(III) salt: Formation of the Au nanowire intermediate and its nonlinear optical properties. J. Phys. Chem. C 2007, 111, 6281–6287. [Google Scholar] [CrossRef]
- Pei, L.; Mori, K.; Adachi, M. Formation process of two-dimensional networked gold nanowires by citrate reduction of AuCl4− and the shape stabilization. Langmuir 2004, 20, 7837–7843. [Google Scholar] [CrossRef] [PubMed]
- Navaladian, S.; Janet, C.M.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. A facile room-temperature synthesis of gold nanowires by oxalate reduction method. J. Phys. Chem. C 2007, 111, 14150–14156. [Google Scholar] [CrossRef]
- Huo, Z.; Tsung, C.K.; Huang, W.; Zhang, X.; Yang, P. Sub-two nanometer single crystal Au nanowires. Nano Lett. 2008, 8, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Peng, H.C.; Zeng, J.; Liu, J.; Gu, Z.; Xia, Y. Facile synthesis of gold wavy nanowires and investigation of their growth mechanism. J. Am. Chem. Soc. 2012, 134, 20234–20237. [Google Scholar] [CrossRef] [PubMed]
- Chirea, M.; Freitas, A.; Vasile, B.S.; Ghitulica, C.; Pereira, C.M.; Silva, F. Gold nanowire networks: Synthesis, characterization, and catalytic activity. Langmuir 2011, 27, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Park, J.C.; Song, H. Polyhedral gold nanocrystals with O h symmetry: From octahedra to cubes. J. Am. Chem. Soc. 2006, 128, 14863–14870. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Yoo, C.I.; Park, J.C.; Park, S.M.; Ryu, S.; Song, H. Directed surface overgrowth and morphology control of polyhedral gold nanocrystals. Angew. Chem. Int. Ed. 2008, 47, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Kim, D.S.; Kim, Z.H.; Lee, Y.W.; Kim, D.; Kim, M.; Kwon, K.; Park, H.J.; Yun, W.S.; Han, S.W. Controlled synthesis and characterization of the enhanced local field of octahedral Au nanocrystals. Chem. Commun. 2008, 0, 6120–6122. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Murphy, C.J. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J. Am. Chem. Soc. 2004, 126, 8648–8649. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gibson, K.J.; Chen, G.; Weizmann, Y. Bipyramid-templated synthesis of monodisperse anisotropic gold nanocrystals. Nat. Commun. 2015, 6, 7571. [Google Scholar] [CrossRef] [PubMed]
- Ah, C.S.; Yun, Y.J.; Park, H.J.; Kim, W.-J.; Ha, D.H.; Yun, W.S. Size-controlled synthesis of machinable single crystalline gold nanoplates. Chem. Mater. 2005, 17, 5558–5561. [Google Scholar] [CrossRef]
- Xin, W.; Severino, J.; De Rosa, I.M.; Yu, D.; McKay, J.; Ye, P.; Yin, X.; Yang, J.M.; Carlson, L.; Kodambaka, S. One-step synthesis of tunable-size gold nanoplates on graphene multilayers. Nano Lett. 2018, 18, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ferhan, A.R.; Gao, Y.; Dandapat, A.; Kim, D.H. High-yield synthesis of triangular gold nanoplates with improved shape uniformity, tunable edge length and thickness. Nanoscale 2014, 6, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Graf, C.; van Blaaderen, A. Metallodielectric colloidal core−shell particles for photonic applications. Langmuir 2002, 18, 524–534. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, J.; Li, L.; Zhao, W.; Li, Y. Synthesis of gold nanoshells through improved seed-mediated growth approach: Brust-like, in situ seed formation. Langmuir 2016, 32, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Chen, C.-H.; Huang, M.H. Seed-mediated synthesis of branched gold nanocrystals derived from the side growth of pentagonal bipyramids and the formation of gold nanostars. Chem. Mater. 2009, 21, 110–114. [Google Scholar] [CrossRef]
- Xie, J.; Lee, J.Y.; Wang, D.I.C. Seedless, surfactantless, high-yield synthesis of branched gold nanocrystals in hepes buffer solution. Chem. Mater. 2007, 19, 2823–2830. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Camargo, P.H.; Xia, Y. Facile synthesis of branched Au nanostructures by templating against a self-destructive lattice of magnetic Fe nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 9653–9656. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Huang, M.H. Synthesis of branched gold nanocrystals by a seeding growth approach. Langmuir 2005, 21, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Liu, M.; Huang, M.H. Direct synthesis of branched gold nanocrystals and their transformation into spherical nanoparticles. J. Phys. Chem. B 2006, 110, 19291–19294. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Z.L.; Ballato, J.; Foulger, S.H.; Carroll, D.L. Monopod, bipod, tripod, and tetrapod gold nanocrystals. J. Am. Chem. Soc. 2003, 125, 16186–16187. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xing, G.; Chu, W.; Liang, X.; Zhao, Y.; Jing, L.; Yuan, H.; Cui, Y.; Dong, J. The growth of complex nanostructures: Synergism of dipolar force and stacking-defects in anisotropic self-assembly. Adv. Mater. 2008, 20, 1794–1798. [Google Scholar] [CrossRef]
- Liao, H.G.; Jiang, Y.X.; Zhou, Z.Y.; Chen, S.P.; Sun, S.G. Shape-controlled synthesis of gold nanoparticles in deep eutectic solvents for studies of structure-functionality relationships in electrocatalysis. Angew. Chem. Int. Ed. 2008, 47, 9100–9103. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Garg, N.; Jin, R. A universal approach to the synthesis of noble metal nanodendrites and their catalytic properties. Angew. Chem. Int. Ed. 2010, 49, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Abalde-Cela, S.; Taladriz-Blanco, P.; de Oliveira, M.G.; Abell, C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci. Rep. 2018, 8, 2440. [Google Scholar] [CrossRef] [PubMed]
- Sajitha, M.; Vindhyasarumi, A.; Gopi, A.; Yoosaf, K. Shape controlled synthesis of multi-branched gold nanocrystals through a facile one-pot bifunctional biomolecular approach. RSC Adv. 2015, 5, 98318–98324. [Google Scholar] [CrossRef]
- Nehl, C.L.; Hafner, J.H. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodriguez-Fernandez, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzan, L.M.; Garcia de Abajo, F.J. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.X.; Kong, X. Review of recent progress of plasmonic materials and nano-structures for surface-enhanced raman scattering. Materials 2015, 8, 3024–3052. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Mackey, M.A.; Huang, X.; Kang, B.; El-Sayed, M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391–3404. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Whang, K.; Shin, Y.; Lee, J.; Kang, T. Synthesis of colloidal plasmonic microspheres via spontaneous formation and three-dimensional assembly of metal nanoparticles. Korean J. Chem. Eng. 2017, 34, 2086–2091. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, J.E.; Jin, S.M.; Lee, J.H.; Lee, I.S.; Yang, I.; Kim, J.S.; Kim, S.K.; Cho, M.H.; et al. Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angew. Chem. Int. Ed. 2006, 45, 7754–7758. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yang, J.; Lee, J.; Haam, S.; Choi, I.H.; Yoo, K.H. Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano 2009, 3, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Won, N.; Jin, H.; Chung, H.; Kim, S. Ph-induced aggregation of gold nanoparticles for photothermal cancer therapy. J. Am. Chem. Soc. 2009, 131, 13639–13645. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Zheng, D.; Zhou, F.; Li, Z.Y.; Li, X.; Xia, Y. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano 2008, 2, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, J.; Kang, J.; Oh, S.J.; Ko, H.J.; Son, J.H.; Lee, K.; Suh, J.S.; Huh, Y.M.; Haam, S. Smart drug-loaded polymer gold nanoshells for systemic and localized therapy of human epithelial cancer. Adv. Mater. 2009, 21, 4339–4342. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Day, D.; Gu, M. Ultra-low energy threshold for cancer photothermal therapy using transferrin-conjugated gold nanorods. Adv. Mater. 2008, 20, 3866–3871. [Google Scholar] [CrossRef]
- Hu, K.W.; Liu, T.M.; Chung, K.Y.; Huang, K.S.; Hsieh, C.T.; Sun, C.K.; Yeh, C.S. Efficient near-IR hyperthermia and intense nonlinear optical imaging contrast on the gold nanorod-in-shell nanostructures. J. Am. Chem. Soc. 2009, 131, 14186–14187. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Yang, M.; Qu, X.; Huai, Y.; Zhu, Y.; Mao, C. Tuning photothermal properties of gold nanodendrites for in vivo cancer therapy within a wide near infrared range by simply controlling their degree of branching. Biomaterials 2016, 104, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Jiang, Y.; Gao, W.; Wang, Y.; Yang, X.; Yang, X.; Liu, Z. Gold nanorods with silica shell and pamam dendrimers for efficient photothermal therapy and low toxic codelivery of anticancer drug and sirna. Adv. Mater. Interfaces 2017, 4, 1701166. [Google Scholar] [CrossRef]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Puia, C.; Agoston-Coldea, L.; Gonciar, D.; Kalman, E.; Zaharie, G.; et al. Selective in vitro photothermal nano-therapy of mrsa infections mediated by igg conjugated gold nanoparticles. Sci. Rep. 2016, 6, 39466. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhang, L.; Zhu, Z.; Jiang, X. Direct facile approach to the fabrication of chitosan-gold hybrid nanospheres. Langmuir 2008, 24, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Dozol, H.; Mériguet, G.; Ancian, B.; Cabuil, V.; Xu, H.; Wang, D.; Abou-Hassan, A. On the synthesis of Au nanoparticles using edta as a reducing agent. J. Phys. Chem. C 2013, 117, 20958–20966. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. The preparation of a gold nanoparticle monolayer on the surface of a polymer inclusion membrane using EDTA as the reducing agent. J. Membr. Sci. 2011, 379, 322–329. [Google Scholar] [CrossRef]
- Lim, T.H.; McCarthy, D.; Hendy, S.C.; Stevens, K.J.; Brown, S.A.; Tilley, R.D. Real-time tem and kinetic monte carlo studies of the coalescence of decahedral gold nanoparticles. ACS Nano 2009, 3, 3809–3813. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Goia, D.; Matijević, E. Tailoring the particle size of monodispersed colloidal gold. Colloids Surf. A 1999, 146, 139–152. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- West, J.L.; Halas, N.J. Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Jung, H.; Cho, H.H.; Lee, J.H.; Nam, Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials 2018, 153, 59–69. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.; Lee, N.; Kim, J.H.; Park, Y.I.; Piao, Y. Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications. Nanomaterials 2018, 8, 252. https://doi.org/10.3390/nano8040252
Jang Y, Lee N, Kim JH, Park YI, Piao Y. Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications. Nanomaterials. 2018; 8(4):252. https://doi.org/10.3390/nano8040252
Chicago/Turabian StyleJang, Youngjin, Nohyun Lee, Jeong Hyun Kim, Yong Il Park, and Yuanzhe Piao. 2018. "Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications" Nanomaterials 8, no. 4: 252. https://doi.org/10.3390/nano8040252
APA StyleJang, Y., Lee, N., Kim, J. H., Park, Y. I., & Piao, Y. (2018). Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications. Nanomaterials, 8(4), 252. https://doi.org/10.3390/nano8040252