An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
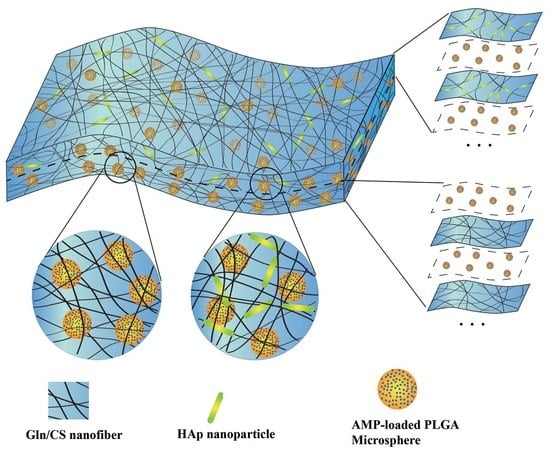
2.2.1. Fabrication of the Nanofibrous Membranes
2.2.2. Characterization of the Nanofibrous Membranes
2.2.3. Cell Culture
2.2.4. Cell Proliferation Assay
2.2.5. Alkaline Phosphatase (ALPase) Activity Assay
2.2.6. Cell Morphological Examination
2.2.7. In Vitro Release Profile of Antimicrobial Peptide
2.2.8. Antibacterial Experiment
2.2.9. Statistical Analysis
3. Results
3.1. Characterization of the Gln/CS Composite Membranes
3.2. In Vitro Biocompatibility
3.3. Alkaline Phosphatase (ALPase) Activity Assay
3.4. In Vitro Drug Release Profile
3.5. Antibacterial Property
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salamanca, E.; Tsai, C.Y.; Pan, Y.H.; Lin, Y.T.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Feng, S.W.; Chang, W.J. In Vitro and In Vivo Study of a Novel Porcine Collagen Membrane for Guided Bone Regeneration. Materials 2016, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Q.; Adayi, A.; Liu, Z.H.; Li, M.; Wu, M.Y.; Xiao, L.H.; Sun, Y.C.; Cai, Q.; Yang, X.P.; Zhang, X.; et al. Asymmetric Collagen/chitosan Membrane Containing Minocycline loaded Chitosan Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2016, 6, 31822. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.J.; He, M.; Niu, Y.Z.; Liu, H.; Crawford, A.; Coates, P.; Chen, D.F.; Shi, R.; Zhang, L.Q. Preparation and in vivo efficient anti-infection property of GTR/GBR implant made by metronidazole loaded electrospun polycaprolactone nanofiber membrane. Int. J. Pharm. 2014, 20, 566–577. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Lopez, M.; Olate, S.; Lanata-Flores, A.; Pozzer, L.; Cavalieri-Pereira, L.; Cantín, M.; Vásquez, B.; de Albergaria-Barbosa, J. New bone formation in a bone defect associated to dental implant using absorbable or non-absorbable membrane in a dog model. Int. J. Clin. Eep. Pathol. 2013, 11, 2292–2299. [Google Scholar]
- Joop, A.; Rahlf, B.; Gellrich, N.C.; Kampmann, A.; von See, C.; Stoetzer, M. Examination of Local Periosteal Microcirculation After Application of Absorbable and Nonabsorbable Membranes. J. Oral Implantol. 2017, 43, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; Zhang, Z.Y.; Xu, R.D.; Cai, P.P.; Kristensen, P.; Chen, M.L.; Huang, Y.D. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J. Biomed. Mater. Res. B Appl. Biomater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Ma, S.Q.; Liu, Z.H.; Geng, H.J.; Lu, X.; Zhang, X.; Li, H.J.; Gao, C.Y.; Zhang, X.; Gao, P. Guided bone regeneration with asymmetric collagen-chitosan membranes containing aspirin-loaded chitosan nanoparticles. Int. J. Nanomed. 2017, 12, 8855–8866. [Google Scholar] [CrossRef] [PubMed]
- Tal, H.; Kozlovsky, A.; Artzi, Z. Long-term bio-degradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration. Clin. Oral Implants Res. 2008, 19, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rikeshwer, P.D.; Gopal, S.B.; Vijay, P.S.; Mohammad, S.Y.; Santosh, P. Design and synthesis of cell selective α/β-diastereomeric peptidomimetic with potent in vivo antibacterial activity against methicillin resistant S. aureus. Bioorg. Chem. 2017, 76, 538–547. [Google Scholar]
- Zhao, P.C.; Xue, Y.; Gao, W.N.; Li, J.H.; Zu, X.Y.; Fu, D.L.; Bai, X.F.; Zuo, Y.J.; Hu, Z.G.; Zhang, F.S. Bacillaceae-derived peptide antibiotics since 2000. Peptides 2017, 101, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Wang, X.J.; Wang, L.N.; Ying, X.X.; Ren, X.; Liu, H.Y.; Xu, L.; Ma, G.W. High in vitro antibacterial activity of Pac-525 against Porphyromonasgingivalis biofilms cultured on titanium. Biomed. Res. Int. 2015, 2015, 909870. [Google Scholar] [PubMed]
- Wei, S.Y.; Wu, J.M.; Kuo, Y.Y.; Chen, H.L.; Yip, B.S.; Tzeng, S.R.; Cheng, J.W. Solution structure of a novel tryptophan-rich peptide with bidirectional antimicrobial activity. J. Bacteriol. 2006, 188, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.B.; Zhou, C.C.; Li, P.; Xu, W.X.; Cao, Y.; Ling, H.; Chen, W.N.; Li, C.M.; Xu, R.; Lamrani, M.; et al. Novel short antibacterial and antifungal peptides with low cytotoxicity: Efficacy and action mechanisms. Biochem. Biophys. Res. Commun. 2010, 398, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Fimland, G.; Eijsink, V.G.; Nissen-Meyer, J. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry 2002, 41, 9508–9515. [Google Scholar] [CrossRef] [PubMed]
- Schibli, D.J.; Epand, R.F.; Vogel, H.J.; Epand, R.M. Tryptophanrich antimicrobial peptides: Comparative properties and membrane interactions. Biochem. Cell Biol. 2002, 80, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.D. Biology of Microorganisms, 2nd ed.; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1974. [Google Scholar]
- Li, Y.; Na, R.; Wang, X.; Liu, H.; Zhao, L.; Sun, X.; Ma, G.; Gui, F. Fabrication of antimicrobial peptide-loaded PLGA/chitosan composite microspheres for long-acting bacterial resistance. Molecules 2017, 22, 1637. [Google Scholar] [CrossRef] [PubMed]
- Jafari, J.; Emami, S.H.; Samadikuchaksaraei, A. Electrospun chitosan-gelatin nanofiberous scaffold: Fabrication and in vitro evaluation. Bio-Med. Mater. Eng. 2011, 21, 99–112. [Google Scholar]
- Wang, S.; Zhao, G. Quantitative characterization of the electrospun gelatin-chitosan nanofibers by coupling scanning electron microscopy and atomic force microscopy. Mater. Lett. 2012, 79, 14–17. [Google Scholar] [CrossRef]
- Yao, S.L.; Liu, H.Y.; Yu, S.K.; Li, Y.Y.; Wang, X.M.; Wang, L.N. Drug-nanoencapsulated PLGA microspheres prepared by emulsion electrospray with controlled released behavior. Regen. Biomater. 2016, 5, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, Y.; Yang, X. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J. Biomed. Mater. Res. A 2009, 90, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Wang, Y.S.; Ma, C. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, W.; Li, J. Preparation of animal polysaccharides nanofibers by electrospinning and their potential biomedical applications. J. Biomed. Mater. Res. A 2015, 103, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mat. Sci. Eng. C Mater. 2014, 45, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef]
- Hood, E.; Simone, E.; Wattamwar, P.; Dziubla, T.; Muzykantov, V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine 2011, 6, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Sebti, T.; Amighi, K. Preparation and in vitro evaluation of lipidic carriers and fillers for inhalation. Eur. J. Pharm. Biopharm. 2006, 63, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bittner, B.; Mader, K.; Kroll, C.; Borchert, H.H.; Kissel, T. Tetracycline-HCl-loaded poly(DL-lactide-co-glycolide) microspheres prepared by a spray drying technique: Influence of gamma-irradiation on radical formation and polymer degradation. J. Control. Release 1999, 59, 23–32. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Q.; Niu, X.; Tan, R.; She, Z. A spheres-in-sphere structure for improving protein-loading poly (lactide-co-glycolide) microspheres. Polym. Degrad. Stab. 2010, 95, 6–13. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Kong, L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun Antibacterial Chitosan-Based Fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chang, J.J.; Lee, Y.H. Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering. Mater. Lett. 2013, 93, 133–136. [Google Scholar] [CrossRef]
- Xue, J.J.; He, M.; Niu, Y.Z.; Crawford, A.; Coates, P.D.; Chen, D.F.; Shi, R.; Zhang, L.Q. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef] [PubMed]







| Test | Type | Barrier Layer | Osteogenic Layer |
|---|---|---|---|
| WCA (°) | - | 73.17 ± 1.22 | 69.53 ± 0.31 |
| Tensile Strength (Mpa) | crosslinked | 5.11 ± 0.96 | 7.22 ± 1.49 |
| un-crosslinked | 3.15 ± 0.57* | 4.97 ± 2.43* |
| Bacterial Species | Inhibition Ratio | Diameter of Inhibition Ring | ||
|---|---|---|---|---|
| 1st wk | 4th wk | 1st wk | 4th wk | |
| E.coli | 94.61% | 68.26% | 11 cm | 9 cm |
| S. aureus | 95.08% | 77.36% | 12 cm | 11 cm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques. Nanomaterials 2018, 8, 327. https://doi.org/10.3390/nano8050327
He Y, Jin Y, Wang X, Yao S, Li Y, Wu Q, Ma G, Cui F, Liu H. An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques. Nanomaterials. 2018; 8(5):327. https://doi.org/10.3390/nano8050327
Chicago/Turabian StyleHe, Yuzhu, Yahui Jin, Xiumei Wang, Shenglian Yao, Yuanyuan Li, Qiong Wu, Guowu Ma, Fuzhai Cui, and Huiying Liu. 2018. "An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques" Nanomaterials 8, no. 5: 327. https://doi.org/10.3390/nano8050327
APA StyleHe, Y., Jin, Y., Wang, X., Yao, S., Li, Y., Wu, Q., Ma, G., Cui, F., & Liu, H. (2018). An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques. Nanomaterials, 8(5), 327. https://doi.org/10.3390/nano8050327