Fabrication and Characterization of Novel Electrothermal Self-Healing Microcapsules with Graphene/Polymer Hybrid Shells for Bitumenious Material
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Prepare of Microcapsules with Graphene
2.3. Characterization of Microcapsules
2.4. Microstructure of Graphene in Shells
2.5. Thermal Resistance of Microcapsules
2.6. Electric Conductivity Measurement
2.7. Thermal Conductivity Measurement
3. Results and Discussion
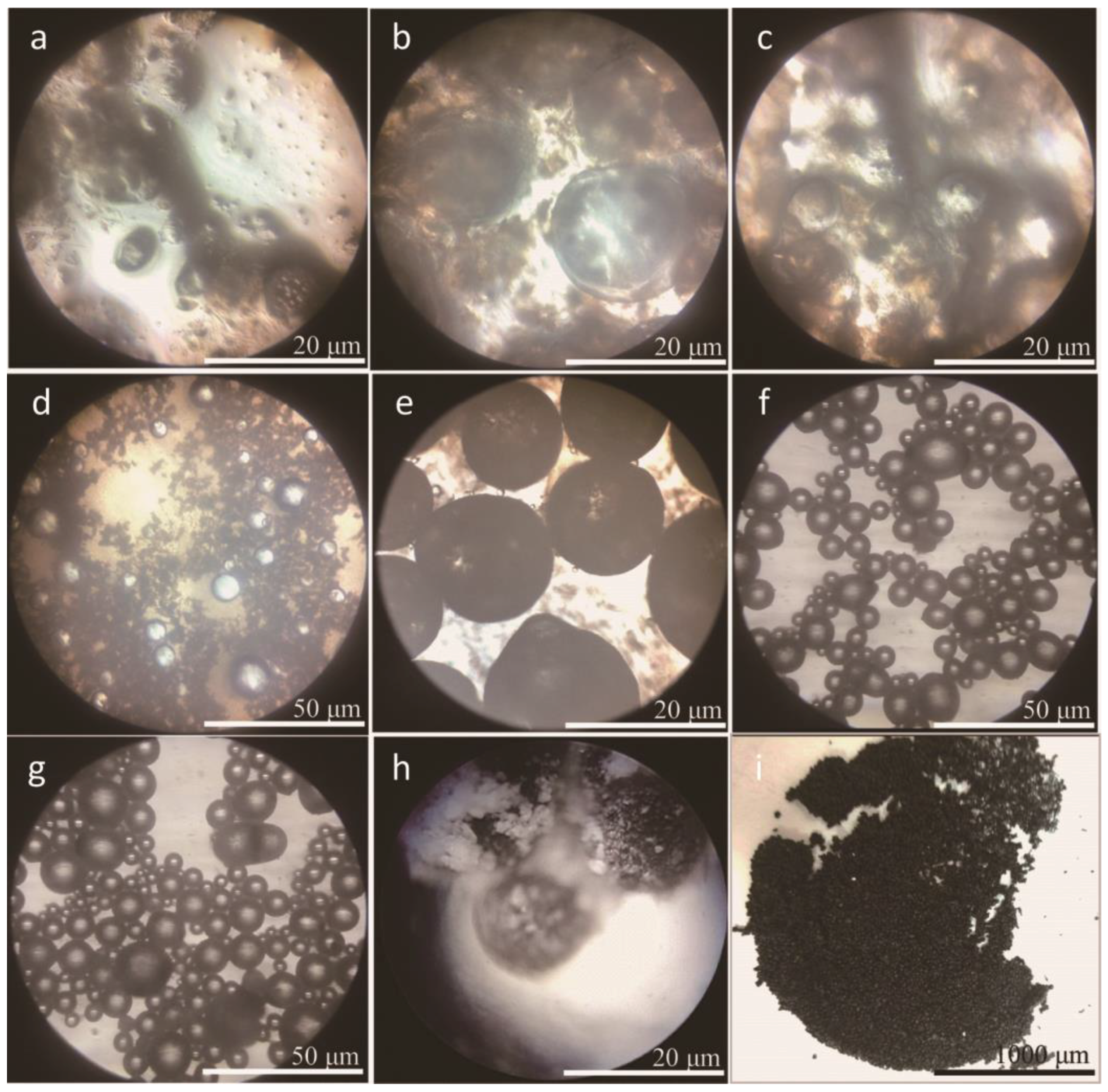
3.1. Morphologies and Geometrical Characteristics
3.2. Chemical Structure of Microcapsule Shells
3.3. Determination of Graphene Deposition in Shells
3.4. Microstructure of Graphene/Organic Hybrid Shells
3.5. Thermal Stability of Microcapsules
3.6. Thermal Conductivity Analysis
3.7. Electric Conductivity Analysis
4. Conclusions
- (1)
- Morphology observations showed that the microcapsules with graphene had a global shape with a compact shell structure. The microcapsules with 2.0–6.0% graphene had a very smooth surface without attachment. The polymer molecules tangled with graphene during the polymerization, so the unconsumed polymer made the tangled graphene to be left in emulsion or only adhere incompactly on shells.
- (2)
- Chemical structure analysis confirmed that the HMMM prepolymer had a cross-linked chemical reaction in emulsion state to yield a compactable shell structure. Graphene did not change the chemical structure of HMMM/graphene composites, it only had a physical bond with cross-linked HMMM networks.
- (3)
- The results proved that the graphene had deposited on the microcapsules surface. The graphene addition had an optimal amount to balance both two actions of charge adsorption and chain entanglement. It was found that the microcapsules had an increasing trend of graphene contents on shells with the increasing of graphene addition during preparation process of microcapsules.
- (4)
- The graphene/HMMM composites structure enhanced the thermal stability of microcapsules. Moreover, the graphene had a good heat transfer performance and made the heat distribution in the microcapsules more uniform, thereby improving the thermal stability of microcapsules.
- (5)
- The thermal conductivity of microcapsules sharply increased to about 8.0 W/m2·K with only 2.0 wt % graphene in shells. At the same time, electrical resistivity of microcapsules/bitumen samples had a decreased trend with more graphene in bitumen.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Amendola, V.; Meneghetti, M. Self-healing at the nanoscale. Nanoscale 2009, 1, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Bergman, S.D.; Wudl, F. Mendable polymers. J. Mater. Chem. 2007, 18, 41–62. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Yin, T.; Rong, M.Z.; Zhang, M.Q. Self healing in polymers and polymer composites. Concepts, realization and outlook: A review. Express Polym. Lett. 2008, 2, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Aissa, B.; Therriault, D.; Haddad, E.; Jamroz, W. Self-healing materials systems: Overview of major approaches and recent developed technologies. Adv. Mater. Sci. Eng. 2012, 10, 1687–1704. [Google Scholar] [CrossRef]
- Fischer, H. Self-repairing material systems: A dream or a reality? Adv. Nat. Sci. 2010, 2, 873–901. [Google Scholar] [CrossRef]
- Steyn, W.J. Potential Applications of nanotechnology in pavement engineering. Transp. Eng. J. ASCE 2010, 18, 14–18. [Google Scholar] [CrossRef]
- Santagata, E.; Baglieri, O.; Tsantilis, L.; Chiappinelli, G. Fatigue and healing properties of nano-reinforced bituminous binders. Int. J. Fatigue 2015, 80, 30–39. [Google Scholar] [CrossRef]
- Mallick, R.B.; Chen, B.L.; Daniel, J.S.; Kandhal, P.S. Heating and its effect on hot in-place recycling of asphalt pavements with rejuvenator. J. Mater. Res. Technol. 2012, 5, 347–359. [Google Scholar]
- Tabaković, A.; Schlangen, E. Self-healing technology for asphalt pavements. Self-Healing Mater. 2015, 273, 285–306. [Google Scholar]
- Shen, S.; Lu, X. Fracture healing properties of asphaltic material under controlled damage. J. Mater. Civ. Eng. 2014, 26, 275–282. [Google Scholar] [CrossRef]
- Butt, A.A.; Birgisson, B.; Kringos, N. Optimizing the highway lifetime by improving the self healing capacity of asphalt. Procedia Soc. Behav. Sci. 2012, 48, 2190–2200. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S. Research on the conductive asphalt concrete’s piezoresistivity effect and its mechanism. Constr. Build. Mater. 2009, 23, 2752–2756. [Google Scholar] [CrossRef]
- Liu, Q.; Schlangen, E.; Garcia, A.; van de Ven, M. Induction heating of electrically conductive porous asphalt concrete. Constr. Build. Mater. 2010, 24, 1207–1213. [Google Scholar] [CrossRef]
- Bhasin, A.; Bommavaram, R.; Greenfield, M.L.; Little, D.N. Use of molecular dynamics to investigate self-healing mechanisms in asphalt binders. J. Mater. Civ. Eng. 2011, 23, 485–492. [Google Scholar] [CrossRef]
- Su, J.F.; Schlangen, E.; Wang, Y.Y. Investigation the self-healing mechanism of aged bitumen using microcapsules containing rejuvenator. Constr. Build. Mater. 2015, 85, 49–56. [Google Scholar] [CrossRef]
- Su, J.F.; Qiu, J.; Schlangen, E.; Wang, Y.Y. Investigation the possibility of a new approach of using microcapsules containing waste cooking oil: In situ, rejuvenation for aged bitumen. Constr. Build. Mater. 2015, 74, 83–92. [Google Scholar] [CrossRef]
- Su, J.F.; Han, S.; Schlangen, E.; Han, N.X.; Liu, B.; Zhang, X.L.; Li, W. Experimental observation of the self-healing microcapsules containing rejuvenator states in asphalt binder. Constr. Build. Mater. 2017, 147, 533–542. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Vickery, J.L.; Patil, A.J.; Mann, S. Fabrication of graphene–polymer nanocomposites with higher-order three-dimensional architectures. Adv. Mater. 2009, 21, 2180–2184. [Google Scholar] [CrossRef]
- Pan, Y.; Bao, H.; Sahoo, N.G.; Wu, T.F.; Li, L. Water-soluble poly(N-isopropylacrylamide) graphene sheets synthesized via click chemistry for drug delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene-metal particle nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Pasricha, R.; Gupta, S.; Srivastava, A.K. A facile and novel synthesis of Ag-graphene-based nanocomposites. Small 2009, 5, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Wang, K.; Luo, S.L.; Tang, Y.H.; Chen, L.Y. Direct electrodeposition of graphene enabling the one-step synthesis of graphene-metal nanocomposite films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Kou, R.; Choi, D.; Yang, Z.G.; Nie, Z.M.; Li, J.; Saraf, L.V.; Hu, D.H.; Zhang, J.G.; Graff, G.L. Ternary self-assembly of ordered metal oxide-graphene nanocomposites for electrochemical energy storage. ACS Nano 2010, 4, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Tao, H.Q.; Yang, K.; Feng, L.Z.; Cheng, L.; Shi, X.Z.; Li, Y.G.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.M.; Keller, M.W.; Moore, J.S.; Sottos, N.R.; White, S.R. Introduction: Self-healing polymers and composites. J. R. Soc. Interface 2007, 4, 347–348. [Google Scholar] [CrossRef]
- Krull, B.P.; Gergely, R.C.R.; Cruz, W.A.S.; Fedonina, Y.I.; Patrick, J.F.; White, S.R.; Sottos, N.R. Strategies for volumetric recovery of large scale damage in polymers. Adv. Funct. Mater. 2016, 26, 4561–4569. [Google Scholar] [CrossRef]
- Desai, S.; Perkins, J.; Harrison, B.S.; Sankar, J. Understanding release kinetics of biopolymer drug delivery microcapsules for biomedical applications. Mater. Sci. Eng. 2010, 168, 127–131. [Google Scholar] [CrossRef]
- Chung, K.; Lee, S.; Park, M.; Yoo, P.; Hong, Y. Preparation and characterization of microcapsule-containing self-healing aasphalt. J. Ind. Eng. Chem. 2015, 29, 330–337. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Lieboldt, M. Permeation of water and gases through cracked textile reinforced concrete. Cem. Concr. Compos. 2011, 33, 725–734. [Google Scholar] [CrossRef]
- Kessler, M.R.; Sottos, N.R.; White, S.R. Self-healing structural composite materials. Compos. Part A-Appl. Sci. Manuf. 2003, 34, 743–753. [Google Scholar] [CrossRef]
- Jones, A.S.; Rule, J.D.; Moore, J.S.; Sottos, N.R.; White, S.R. Life extension of self-healing polymers with rapidly growing fatigue cracks. J. R. Soc. Interface 2007, 4, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Liang, G.Z.; Xie, J.Q.; Lim, L.; Guo, J. Preparation and characterization of poly (urea-formaldehyde) microcapsules filled with epoxy resins. Polymer 2006, 47, 5338–5349. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; Mcilroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Wang, J.P.; Zhao, X.P.; Guo, H.L.; Zheng, Q. Preparation of microcapsules containing two-phase core materials. Langmuir 2004, 20, 10845–10850. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q.; Chen, J.; Yang, G.C.; Li, X.M. Self-healing polymeric materials using epoxy/mercaptan as the healant. Macromolecules 2008, 41, 5197–5202. [Google Scholar] [CrossRef]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of isocyanates for self-healing polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Su, J.F.; Schlangen, E.; Han, N.X.; Han, S.; Li, W. Fabrication and characterization of self-healing microcapsules containing bituminous rejuvenator by a nano-inorganic/organic hybrid method. Constr. Build. Mater. 2016, 121, 471–482. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Bayer, B.C.; Raoul, B.; Wang, Z.J.; Baehtz, C.; Weatherup, R.S.; Willinger, M.G.; Schloeql, R.; Hofmann, S. Observing graphene grow: Catalyst–graphene interactions during scalable graphene growth on polycrystalline copper. Nano Lett. 2013, 13, 4769–4778. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.M.; Hu, S.F.; Zhang, R.; Xu, C.C.; Zhang, F.; Liu, Q.T. Enhanced electrical properties of graphite/ABS composites prepared via supercritical CO2 processing. Polym. Bull. 2017, 74, 4279–4295. [Google Scholar] [CrossRef]
- Al-Attabi, N.Y.; Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Evans, M.; Gunatillake, P. Preparation and characterization of highly conductive polyurethane composites containing graphene and gold nanoparticles. J. Mater. Sci. 2017, 52, 11774–11784. [Google Scholar] [CrossRef]











| Microcapsules Sample | Core/Shell Weight Ratio | Graphene /Shell (wt %) | Emulsion Rate (r·min−1) | Mean Size (μm) | Shell Thickness (μm) |
|---|---|---|---|---|---|
| MG-0 | 1/1 | 0 | 3000 | 22.4 | 2.5 |
| MG-2 | 1/1 | 2 | 3000 | 22.5 | 2.4 |
| MG-4 | 1/1 | 4 | 3000 | 22.9 | 2.5 |
| MG-6 | 1/1 | 6 | 3000 | 23.4 | 2.6 |
| MG-8 | 1/1 | 8 | 3000 | 24.3 | 2.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, Y.; Su, J.; Zhang, X.; Wang, Y.; Tan, Y. Fabrication and Characterization of Novel Electrothermal Self-Healing Microcapsules with Graphene/Polymer Hybrid Shells for Bitumenious Material. Nanomaterials 2018, 8, 419. https://doi.org/10.3390/nano8060419
Wang X, Guo Y, Su J, Zhang X, Wang Y, Tan Y. Fabrication and Characterization of Novel Electrothermal Self-Healing Microcapsules with Graphene/Polymer Hybrid Shells for Bitumenious Material. Nanomaterials. 2018; 8(6):419. https://doi.org/10.3390/nano8060419
Chicago/Turabian StyleWang, Xinyu, Yandong Guo, Junfeng Su, Xiaolong Zhang, Yingyuan Wang, and Yiqiu Tan. 2018. "Fabrication and Characterization of Novel Electrothermal Self-Healing Microcapsules with Graphene/Polymer Hybrid Shells for Bitumenious Material" Nanomaterials 8, no. 6: 419. https://doi.org/10.3390/nano8060419
APA StyleWang, X., Guo, Y., Su, J., Zhang, X., Wang, Y., & Tan, Y. (2018). Fabrication and Characterization of Novel Electrothermal Self-Healing Microcapsules with Graphene/Polymer Hybrid Shells for Bitumenious Material. Nanomaterials, 8(6), 419. https://doi.org/10.3390/nano8060419




