Surface Sizing Treated MWCNTs and Its Effect on the Wettability, Interfacial Interaction and Flexural Properties of MWCNT/Epoxy Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
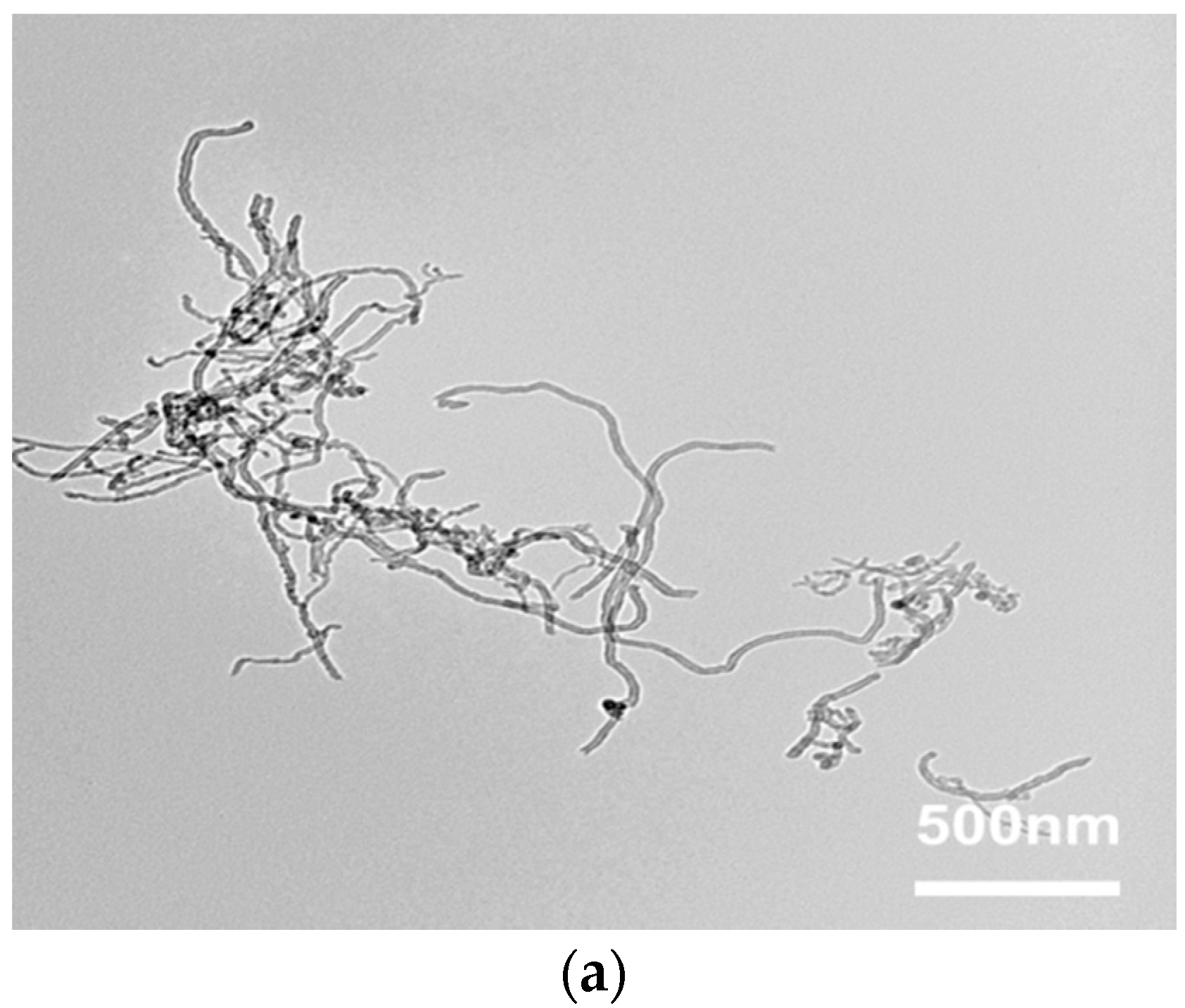
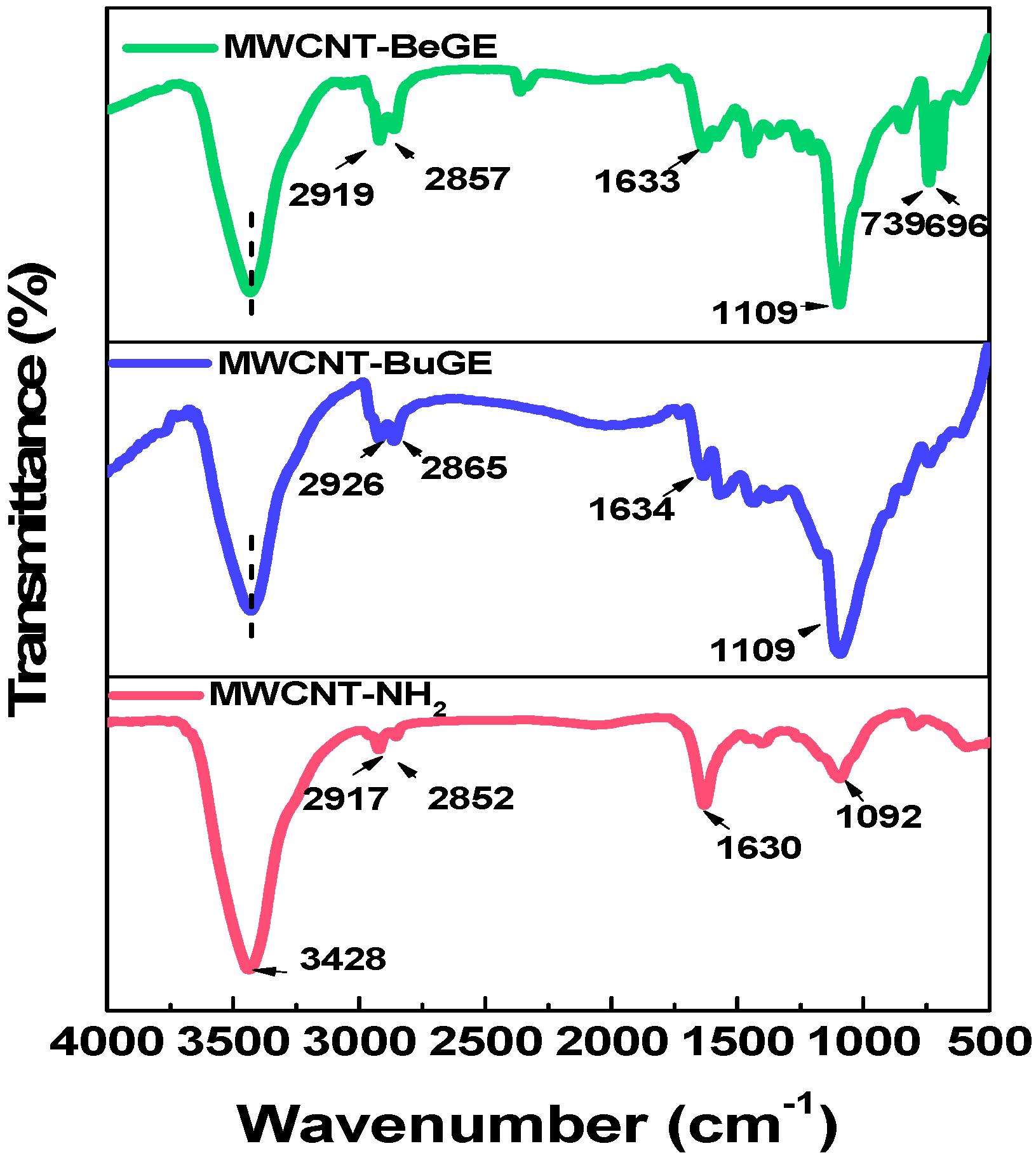
3.1. Surface Characterization of MWCNTs
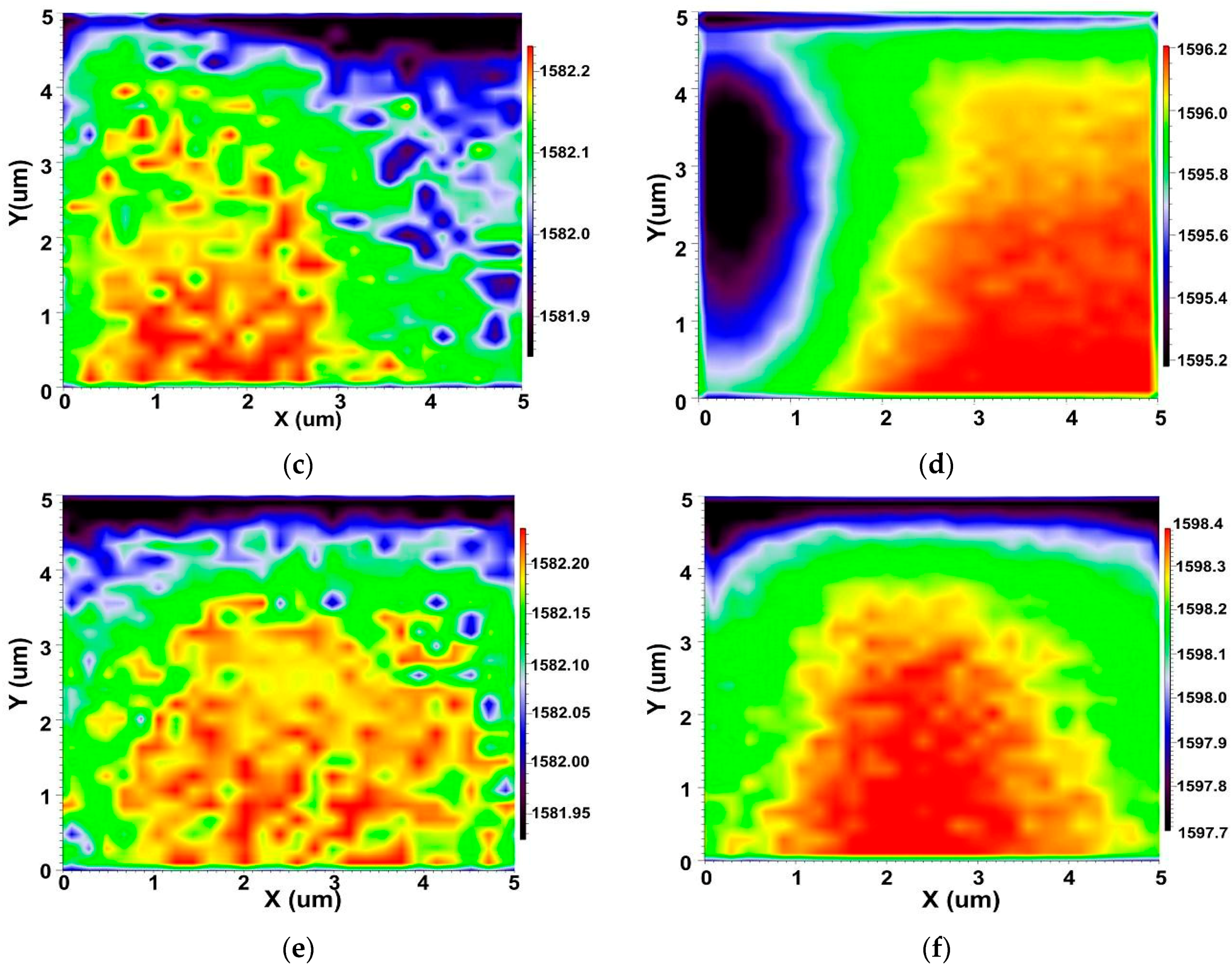
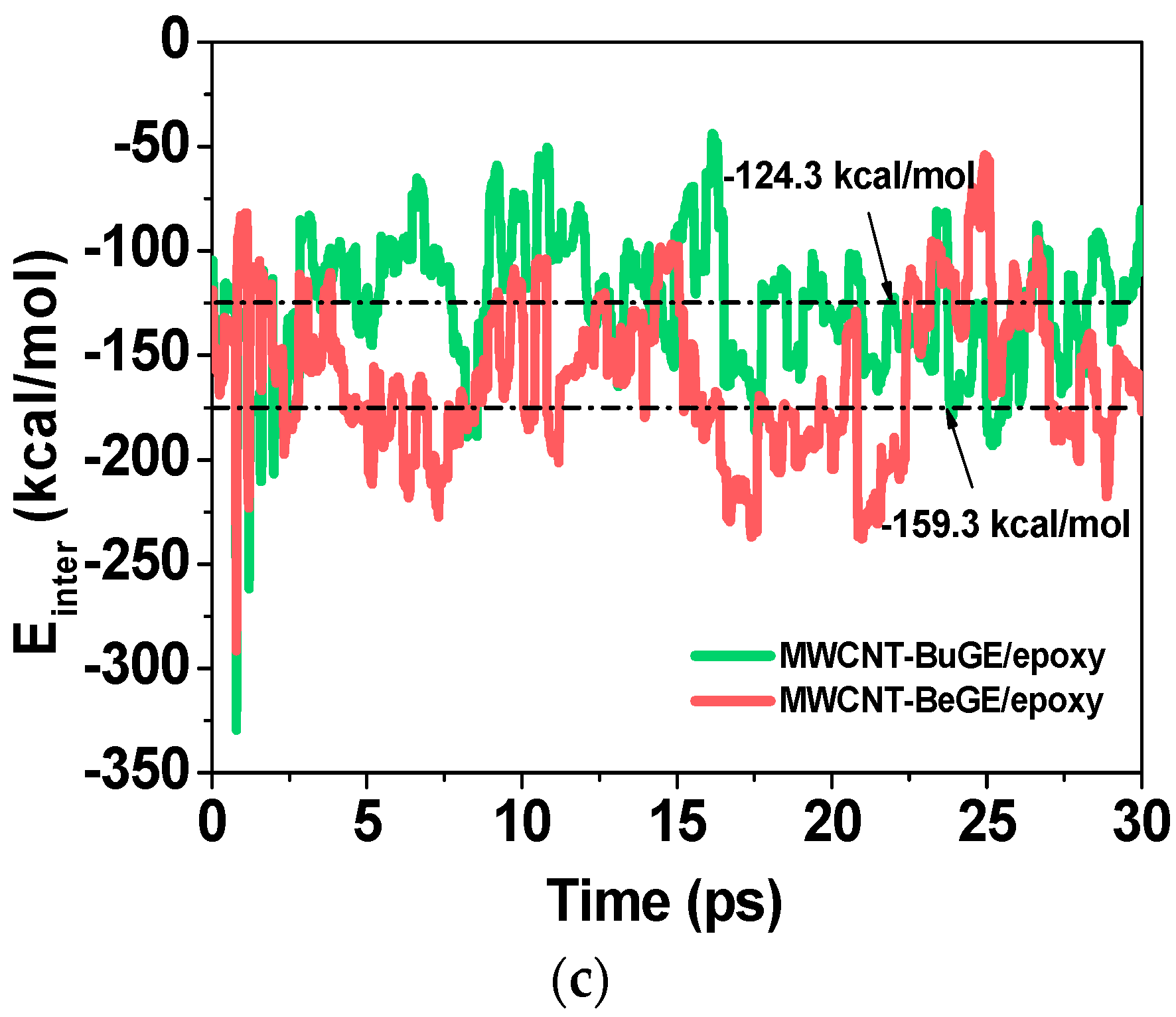
3.2. Interfacial Analysis of MWCNT/Epoxy Nanocomposite
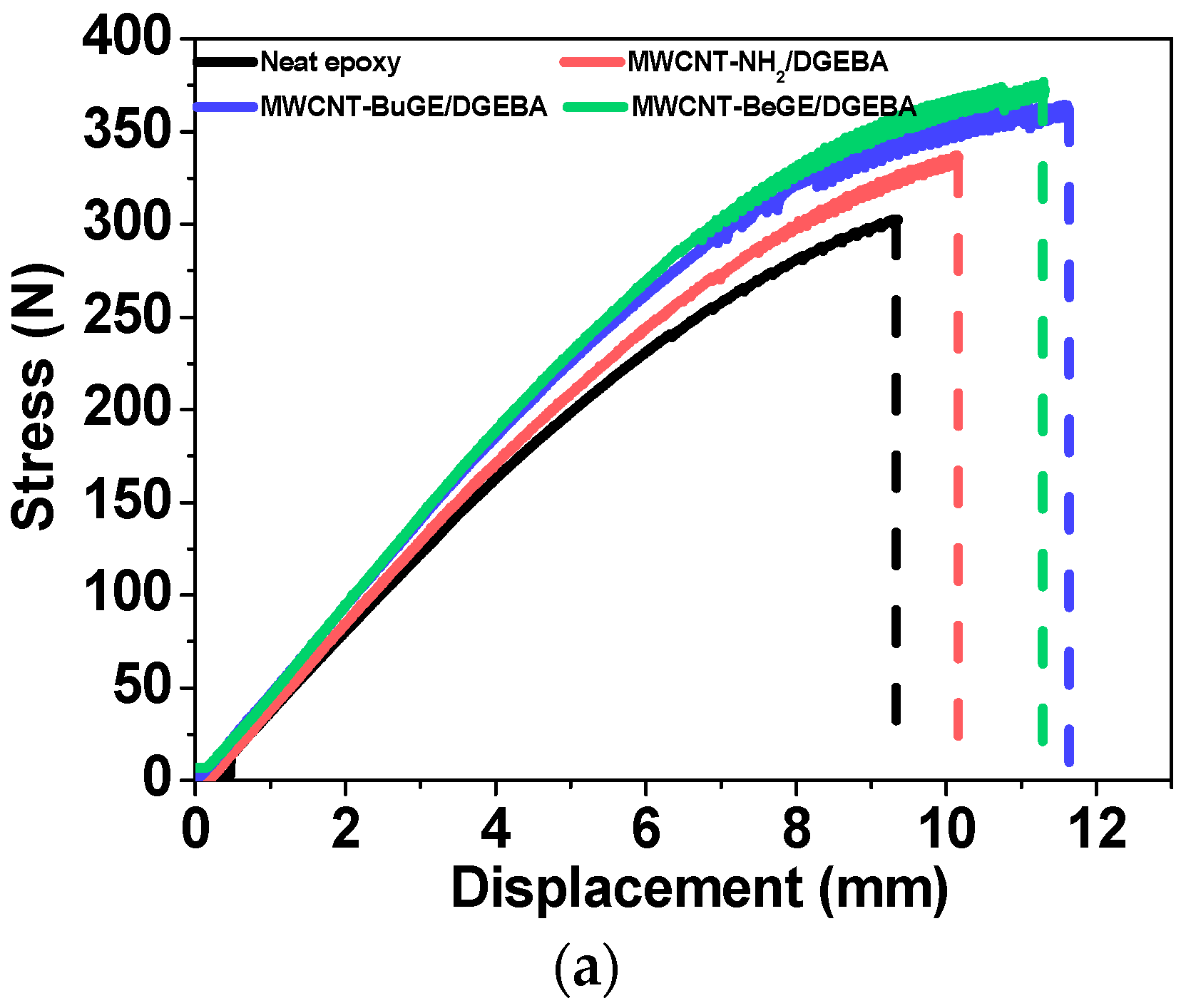
3.3. Fracture Morphology and Mechanical Analysis of MWCNT/Epoxy Nanocomposite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ricciardi, M.R.; Papa, I.; Langella, A.; Langella, T.; Lopresto, V.; Antonucci, V. Mechanical properties of glass fibre composites based on nitrile rubber toughened modified epoxy resin. Compos. Part B Eng. 2018, 139, 259–267. [Google Scholar] [CrossRef]
- Chung, D.D.L. Processing-structure-property relationships of continuous carbon fiber polymer-matrix composites. Mater. Sci. Eng. R 2017, 113, 1–29. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Sainsbury, T.; Liaghat, G.H.; Vahid, S. Improving the fracture toughness and the strength of epoxy using nanomaterials—A review of the current status. Nanoscale 2015, 7, 10294–10329. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chevali, V.S.; Xu, Z.G.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. Part B Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Cui, M.J.; Ren, S.M.; Qiu, S.H.; Zhao, H.C.; Wang, L.P.; Xue, Q.J. Non-covalent functionalized multi-wall carbon nanotubes filled epoxy composites: Effect on corrosion protection and tribological performance. Surf. Coat. Technol. 2018, 340, 74–85. [Google Scholar] [CrossRef]
- Quan, D.; Urdániz, J.L.; Ivanković, A. Enhancing mode-I and mode-II fracture toughness of epoxy and carbon fibre reinforced epoxy composites using multi-walled carbon nanotubes. Mater. Des. 2018, 143, 81–92. [Google Scholar] [CrossRef]
- Garate, H.; Bianchi, M.; Pietrasanta, L.I.; Goyanes, S.; D’Accorso, N.B. High-energy dissipation performance in epoxy coatings by the synergistic effect of carbon nanotube/block copolymer conjugates. ACS Appl. Mater. Interfaces 2017, 9, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Zhang, L.Y.; Zheng, M.; Park, C.; Wang, X.Q.; Ke, C.H. Quantitative nanomechanical characterization of the van der Waals interfaces between carbon nanotubes and epoxy. Carbon 2015, 82, 214–228. [Google Scholar] [CrossRef]
- Owuor, P.S.; Yang, Y.; Kaji, T.; Koizumi, R.; Ozden, S.; Vajtai, R.; Lou, J.; Penev, E.S.; Yakobson, B.I.; Tiwary, C.S.; et al. Enhancing mechanical properties of nanocomposites using interconnected carbon nanotubes (iCNT) as reinforcement. Adv. Eng. Mater. 2017, 19, 1600499. [Google Scholar] [CrossRef]
- Awad, S.A.; Fellows, C.M.; Mahini, S.S. Effects of accelerated weathering on the chemical, mechanical, thermal and morphological properties of an epoxy/multi-walled carbon nanotube composite. Polym. Test. 2018, 66, 70–77. [Google Scholar] [CrossRef]
- Li, Q.T.; Jiang, M.J.; Wu, G.; Chen, L.; Chen, S.C.; Cao, Y.X.; Wang, Y.Z. Photothermal conversion triggered precisely targeted healing of epoxy resin based on thermoreversible diels−alder network and amino-functionalized carbon nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 20797–20807. [Google Scholar] [CrossRef] [PubMed]
- Sydlik, S.A.; Lee, J.H.; Walish, J.J.; Thomas, E.L.; Swager, T.M. Epoxy functionalized multi-walled carbon nanotubes for improved adhesives. Carbon 2013, 59, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Stylianakis, M.M.; Mikroyannidis, J.A.; Kymakis, E. A facile, covalent modification of single-wall carbon nanotubes by thiophene for use in organic photovoltaic cells. Sol. Energy Mater. Sol. Cells 2010, 94, 267–274. [Google Scholar] [CrossRef]
- Le, V.T.; Ngo, C.L.; Le, Q.T.; Ngo, T.T.; Nguyen, D.N.; Vu, M.T. Surface modification and functionalization of carbon nanotube with some organic compounds. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035017. [Google Scholar] [CrossRef] [Green Version]
- Sablok, K.; Bhalla, V.; Sharma, P.; Kausha, R.; Chaudhary, S.; Suri, C.R. Amine functionalized graphene oxide/CNT nanocomposite for ultrasensitive electrochemical detection of trinitrotoluene. J. Hazard. Mater. 2013, 248–249, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Mashhadzadeha, A.H.; Fereidoona, A.; Ahangari, M.G. Surface modification of carbon nanotubes using 3-aminopropyltriethoxysilane to improve mechanical properties of nanocomposite based polymer matrix: Experimental and Density functional theory study. Appl. Surf. Sci. 2017, 420, 167–179. [Google Scholar] [CrossRef]
- Sharma, K.; Kaushalyayan, K.S.; Shukla, M. Pull-out simulations of interfacial properties of amine functionalized multi-walled carbon nanotube epoxy composites. Comput. Mater. Sci. 2015, 99, 232–241. [Google Scholar] [CrossRef]
- Liu, Y.; Wilkinson, A. Rheological percolation behaviour and fracture properties of nanocomposites of MWCNTs and a highly crosslinked aerospace-grade epoxy resin system. Compos. Part A Appl. Sci. Manuf. 2018, 105, 97–107. [Google Scholar] [CrossRef]
- Alam, A.; Wan, C.Y.; McNally, T. Surface amination of carbon nanoparticles for modification of epoxy resins: Plasma-treatment vs. wet-chemistry approach. Eur. Polym. J. 2017, 87, 422–448. [Google Scholar] [CrossRef]
- Rezazadeh, V.; Pourhossaini, M.R.; Salimi, A. Effect of amine-functionalized dispersant on cure and electrical properties of carbon nanotube/epoxy nanocomposites. Prog. Org. Coat. 2017, 111, 389–394. [Google Scholar] [CrossRef]
- Khare, K.S.; Khabaz, F.; Khare, R. Effect of carbon nanotube functionalization on mechanical and thermal properties of cross-linked epoxy−carbon nanotube nanocomposites: Role of strengthening the interfacial interactions. ACS Appl. Mater. Interfaces 2014, 6, 6098–6110. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Jun, G.H.; Park, J.K.; Kim, J.C.; Ryu, H.J.; Hong, S.H. Improvement of modulus, strength and fracture toughness of CNT/Epoxy nanocomposites through the functionalization of carbon nanotubes. Compos. Part B Eng. 2017, 129, 169–179. [Google Scholar] [CrossRef]
- Lavorgna, M.; Romeo, V.; Martone, A.; Zarrelli, M.; Giordano, M.; Buonocore, G.G.; Qu, M.Z.; Fei, G.X.; Xia, H.S. Silanization and silica enrichment of multiwalled carbon nanotubes: Synergistic effects on the thermal-mechanical properties of epoxy nanocomposites. Eur. Polym. J. 2013, 49, 428–438. [Google Scholar] [CrossRef]
- Ma, P.C.; Mo, S.Y.; Tang, B.Z.; Kim, J.K. Dispersion, interfacial interaction and re-agglomeration of functionalized carbon nanotubes in epoxy composites. Carbon 2012, 48, 1824–1834. [Google Scholar] [CrossRef]
- Martinez-Rubi, Y.; Ashrafi, B.; Guan, J.; Kingston, C.; Johnston, A.; Simanrd, B.; Mirjalili, V.; Hubert, P.; Deng, L.B.; Young, R.J. Toughening of epoxy matrices with reduced single-walled carbon nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 2309–2326. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.W.; Cai, Y.M.; Liu, W.B.; Yang, F.; Jiang, L.; Jiao, W.C.; Wang, R.G. Preparation of carbon fiber unsaturated sizing agent for enhancing interfacial strength of carbon fiber/vinyl ester resin composite. Appl. Surf. Sci. 2018, 439, 88–95. [Google Scholar] [CrossRef]
- Chang, C.M.; Liu, Y.L. Functionalization of multi-walled carbon nanotubes with non-reactive polymers through an ozone-mediated process for the preparation of a wide range of high performance polymer/carbon nanotube composites. Carbon 2010, 48, 1289–1297. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Murugesan, S.; Myers, K.; Subramanian, V. Amino-functionalized and acid treated multi-walled carbon nanotubes as supports for electrochemical oxidation of formic acid. Appl. Catal. B Environ. 2011, 103, 266–274. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.J.; Gao, L.; Zhong, W.H.; Sui, G.; Yang, X.P. Significantly improved electrical and interlaminar mechanical properties of carbon fiber laminated composites by using special carbon nanotube pre-dispersion mixture. Compos. Part A Appl. Sci. Manuf. 2017, 95, 294–303. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc phthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Bibi, S.; Yasin, T.; Nawaz, M.; Price, G.J. Comparative study of the modification of multi-wall carbon nanotubes by gamma irradiation and sonochemically assisted acid etching. Mater. Chem. Phys. 2018, 207, 23–29. [Google Scholar] [CrossRef]
- Maleki, A.; Hamesadeghi, U.; Daraei, H.; Hayati, B.; Najafi, F.; McKay, G.; Rezaee, R. Amine functionalized multi-walled carbon nanotubes: Single and binary systems for high capacity dye removal. Chem. Eng. J. 2017, 313, 826–835. [Google Scholar] [CrossRef]
- Kang, W.S.; Rhee, K.Y.; Park, S.J. Influence of surface energetics of graphene oxide on fracture toughness of epoxy nanocomposites. Compos. Part B Eng. 2017, 114, 175–183. [Google Scholar] [CrossRef]
- Zhao, M.; Meng, L.H.; Ma, L.C.; Ma, L.N.; Yang, X.B.; Huang, Y.D.; Ryu, J.E.; Shankar, A.; Li, T.X.; Yan, C.; et al. Layer-by-layer grafting CNTs onto carbon fibers surface for enhancing the interfacial properties of epoxy resin composites. Compos. Sci. Technol. 2018, 154, 28–36. [Google Scholar] [CrossRef]
- Wang, S.R.; Liang, R.; Wang, B.; Zhang, C. Load-transfer in functionalized carbon nanotubes/polymer composites. Chem. Phys. Lett. 2008, 457, 371–375. [Google Scholar] [CrossRef]
- Yu, B.; Fu, S.; Wu, Z.Q.; Bai, H.W.; Ning, N.Y.; Fu, Q. Molecular dynamics simulations of orientation induced interfacial enhancement between single walled carbon nanotube and aromatic polymers chains. Compos. Part A Appl. Sci. Manuf. 2015, 73, 155–165. [Google Scholar] [CrossRef]
- Qu, Z.H.; Wang, G.J. A comparative study on the properties of the different amino-functionalized multiwall carbon nanotubes reinforced epoxy resin composites. J. Appl. Polym. Sci. 2012, 124, 403–408. [Google Scholar]
- Yang, K.; Gu, M.Y.; Guo, Y.P.; Pan, X.F.; Mu, G.H. Effects of carbon nanotube functionalization on the mechanical and thermal properties of epoxy composites. Carbon 2009, 47, 1723–1737. [Google Scholar] [CrossRef]
- Wu, F.; Zheng, Y.P.; Qu, P.; Wang, N.; Chen, L.X. A liquid-like multiwalled carbon nanotube derivative and its epoxy nanocomposites. J. Appl. Polym. Sci. 2013, 130, 2217–2224. [Google Scholar] [CrossRef]













| Samples | C–N–C (%) | C–NH2 (%) | –NH–C=O (%) |
|---|---|---|---|
| MWCNT-NH2 | 31.4 | 35.0 | 33.6 |
| MWCNT-BuGE | 38.0 | 33.5 | 28.5 |
| MWCNT-BeGE | 36.7 | 31.9 | 31.4 |
| CNT Type | Content (wt.%) | Flexural Strength/Mpa (Increase, %) | Flexural Modulus/GPa (Increase, %) | Reference | ||
|---|---|---|---|---|---|---|
| Control Sample | Composites | Control Sample | Composites | |||
| MWCNT-NH2 | 0.5 | 107 | 128 (19.6%) | 2.92 | 3.51 (20.2%) | [25] |
| MWCNT-NH2 | 0.5 | 90 | 102 (13.3%) | 1.88 | 2.21 (17.6%) | [39] |
| MWCNT-NH2 | 0.6 | 86 | 109 (26.7%) | 2.21 | 2.69 (21.7%) | [40] |
| MWCNT derivatives | 1 | 109 | 122 (12.1%) | - | - | [41] |
| MWCNT-NH2 | 0.5 | 122 | 135 (10.7%) | 2.62 | 3.22 (22.9%) | This study |
| MWCNT-BuGE | 0.5 | 122 | 141 (15.6%) | 2.62 | 3.41 (30.1%) | This study |
| MWCNT-BeGE | 0.5 | 122 | 150 (22.9%) | 2.62 | 3.61 (37.8%) | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhao, X.; Sui, G.; Yang, X. Surface Sizing Treated MWCNTs and Its Effect on the Wettability, Interfacial Interaction and Flexural Properties of MWCNT/Epoxy Nanocomposites. Nanomaterials 2018, 8, 680. https://doi.org/10.3390/nano8090680
Zhang Q, Zhao X, Sui G, Yang X. Surface Sizing Treated MWCNTs and Its Effect on the Wettability, Interfacial Interaction and Flexural Properties of MWCNT/Epoxy Nanocomposites. Nanomaterials. 2018; 8(9):680. https://doi.org/10.3390/nano8090680
Chicago/Turabian StyleZhang, Qingjie, Xinfu Zhao, Gang Sui, and Xiaoping Yang. 2018. "Surface Sizing Treated MWCNTs and Its Effect on the Wettability, Interfacial Interaction and Flexural Properties of MWCNT/Epoxy Nanocomposites" Nanomaterials 8, no. 9: 680. https://doi.org/10.3390/nano8090680
APA StyleZhang, Q., Zhao, X., Sui, G., & Yang, X. (2018). Surface Sizing Treated MWCNTs and Its Effect on the Wettability, Interfacial Interaction and Flexural Properties of MWCNT/Epoxy Nanocomposites. Nanomaterials, 8(9), 680. https://doi.org/10.3390/nano8090680





