Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Application on the Detection of Human and Animal Original Biomolecule
2.1. Protein
2.1.1. Crucial Functional Proteins
2.1.2. Disease Biomarkers
2.2. Nucleic Acid
2.2.1. DNA
2.2.2. RNA
2.3. Carbohydrate
2.4. Others
3. Application on the Detection of Plant Original Biomolecules
3.1. Lipids and Antioxidant
3.2. Anthocyanins
3.3. Chinese Herbal Medicinal Ingredients
3.4. Molecular Fingerprint Identification of Plant
3.5. Other Plant Components
4. Application on the Detection of Microorganism Original Biomolecules
4.1. Bacteria Original Biomolecules
4.1.1. Bacteria DNAs
4.1.2. Bacteria Proteins
4.1.3. Other Bacteria Component Molecules
4.1.4. Bacteria Metabolites
4.2. Virus Original Biomolecules
4.2.1. Virus Proteins
4.2.2. Virus DNAs
4.3. Other Microorganism Original Biomolecules
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.; Yang, H.J.; Liu, D.C.; He, H.B.; Wang, C.F.; Zhong, J.F.; Gao, Y.D.; Zeng, Y.J. Analysis of biofilms formation and associated genes detection in staphylococcus isolates from bovine mastitis. Int. J. Appl. Res. Vet. Med. 2012, 10, 62–68. [Google Scholar] [CrossRef]
- Xie, S.B.; Zhou, J. Harnessing plant biodiversity for the discovery of novel anticancer drugs targeting microtubules. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, J.; Feng, Z.; Wang, F.Y.; Xie, S.S.; Bu, S.Z. Immunoassay for tumor markers in human serum based on si nanoparticles and sic@ag sers-active substrate. Analyst 2016, 141, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Q.; Han, L.; Du, S.; Yu, H.; Zhang, H. Rapid and sensitive detection of salmonella typhimurium based on the photothermal effect of magnetic nanomaterials. Sens. Actuators B Chem. 2018, 268, 188–194. [Google Scholar] [CrossRef]
- Rombout, J.; Yang, G.W.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, N.; Wang, Y.; Liu, S.S.; Yang, Z.; Wang, F.; Wan, S.B. Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hanif, S.; Liu, H.L.; Chen, M.; Muhammad, P.; Zhou, Y.; Cao, J.; Ahmed, S.A.; Xu, J.J.; Xia, X.H.; Chen, H.Y.; et al. Organic cyanide decorated sers active nanopipettes for quantitative detection of hemeproteins and fe3+ in single cells. Anal. Chem. 2017, 89, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Robles, G.; Lopez, P.; Bach, S.B.H.; Alvarez, M.; Whetten, R.L. Liquid chromatography separation and mass spectrometry detection of silver-lipoate ag29(la)12 nanoclusters: Evidence of isomerism in the solution phase. Anal. Chem. 2018, 90, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lv, J.; Liu, X.; Tang, Z.; Wang, X.; Xu, Y.; Hammock, B.D. Development of a nanobody-avitag fusion protein and its application in a streptavidin-biotin-amplified enzyme-linked immunosorbent assay for ochratoxin a in cereal. Anal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Feng, Z.T.; Yuan, F.; Guo, J.R.; Suo, S.S.; Wang, B.S. Identification and functional analysis of the autofluorescent substance in limonium bicolor salt glands. Plant Physiol. Biochem. 2015, 97, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Chen, M.; Leng, B.Y.; Wang, B.S. An efficient autofluorescence method for screening limonium bicolor mutants for abnormal salt gland density and salt secretion. S. Afr. J. Bot. 2013, 88, 110–117. [Google Scholar] [CrossRef]
- Guo, F.D.; Liu, C.L.; Xia, H.; Bi, Y.P.; Zhao, C.Z.; Zhao, S.Z.; Hou, L.; Li, F.G.; Wang, X.J. Induced expression of atlec1 and atlec2 differentially promotes somatic embryogenesis in transgenic tobacco plants. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Yang, T.Y.; Li, C.S.; Jin, L.L.; Lou, H.; Song, Y.T. Prenatal detection of thalassemia by cell-free fetal DNA (cffdna) in maternal plasma using surface enhanced raman spectroscopy combined with pcr. Biomed. Opt. Express 2018, 9, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Xiong, Y.; Tang, W.Y.; Guo, Y.; Yan, X.H.; Si, M.Z. Near-infrared surface-enhanced raman spectroscopy (nir-sers) studies on oxyheamoglobin (oxyhb) of liver cancer based on pva-ag nanofilm. J. Raman Spectrosc. 2013, 44, 362–369. [Google Scholar] [CrossRef]
- Casella, M.; Lucotti, A.; Tommasini, M.; Bedoni, M.; Forvi, E.; Gramatica, F.; Zerbi, G. Raman and sers recognition of beta-carotene and haemoglobin fingerprints in human whole blood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.Q.; Wang, M.H.; Petti, L.; Jiang, T.; Jia, Z.H.; Xie, S.S.; Zhou, J. Sers-based multiplex immunoassay of tumor markers using double sio2@ag immune probes and gold-film hemisphere array immune substrate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 48–58. [Google Scholar] [CrossRef]
- Liu, N.N.; Chi, Y.N.; Dong, L.H.; Li, J.H.; Shan, G.Y.; Chen, Y.W.; Liu, Y.C. Label-free detection of bovine serum albumin protein based on sio2/au nanoshells as near-infrared surface-enhanced raman spectroscopy nanoprobe. J. Nanosci. Nanotechnol. 2016, 16, 7103–7109. [Google Scholar] [CrossRef]
- Liang, A.H.; Li, C.N.; Wang, X.L.; Luo, Y.H.; Wen, G.Q.; Jiang, Z.L. Immunocontrolling graphene oxide catalytic nanogold reaction and its application to sers quantitative analysis. ACS Omega 2017, 2, 7349–7358. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.B.; He, J.H.; Li, J.; Zhang, Y. Lotus seedpod inspired sers substrates: A novel platform consisting of 3d sub-10 nm annular hot spots for ultrasensitive sers detection. Adv. Opt. Mater. 2018, 6. [Google Scholar] [CrossRef]
- Yang, Y.J.; Jiang, X.Y.; Chao, J.; Song, C.Y.; Liu, B.; Zhu, D.; Sun, Y.Z.; Yang, B.Y.; Zhang, Q.W.; Chen, Y.; et al. Synthesis of magnetic core-branched au shell nanostructures and their application in cancer-related mirna detection via sers. Sci. China Mater. 2017, 60, 1129–1144. [Google Scholar] [CrossRef]
- Karadan, P.; Aggarwal, S.; Anappara, A.A.; Narayana, C.; Barshilia, H.C. Tailored periodic si nanopillar based architectures as highly sensitive universal sers biosensing platform. Sens. Actuators B Chem. 2018, 254, 264–271. [Google Scholar] [CrossRef]
- David, C.; Guillot, N.; Shen, H.; Toury, T.; de la Chapelle, M.L. Sers detection of biomolecules using lithographed nanoparticles towards a reproducible sers biosensor. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restaino, S.M.; White, I.M. Inkjet-printed paper surface enhanced raman spectroscopy (sers) sensors: Portable, low cost diagnostics for microrna. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Li, L.; Liu, C.; Cao, X.W.; Wang, Y.; Dong, J.; Qian, W.P. Determination of carcinoembryonic antigen by surface-enhanced raman spectroscopy using gold nanobowl arrays. Anal. Lett. 2017, 50, 982–998. [Google Scholar] [CrossRef]
- Yang, L.Y.; Fu, C.C.; Wang, H.L.; Xu, S.P.; Xu, W.Q. Aptamer-based surface-enhanced raman scattering (sers) sensor for thrombin based on supramolecular recognition, oriented assembly, and local field coupling. Anal. Bioanal. Chem. 2017, 409, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Foti, A.; D’Andrea, C.; Villari, V.; Micali, N.; Donato, M.G.; Fazio, B.; Marago, O.M.; Gillibert, R.; de la Chapelle, M.L.; Gucciardi, P.G. Optical aggregation of gold nanoparticles for sers detection of proteins and toxins in liquid environment: Towards ultrasensitive and selective detection. Materials 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Ren, K.N.; Zhao, Y.H.; Dai, W.; Wu, H.K. Convenient formation of nanoparticle aggregates on microfluidic chips for highly sensitive sers detection of biomolecules. Anal. Bioanal. Chem. 2012, 402, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.P.; Si, M.Z.; Zhu, Y.Q.; Miao, L.; Xu, G. Surface-enhanced raman scattering (sers) spectra of hemoglobin of mouse and rabbit with self-assembled nano-silver film. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 177–180. [Google Scholar] [CrossRef] [PubMed]
- He, J.C.; Li, G.K.; Hu, Y.L. Aptamer recognition induced target-bridged strategy for proteins detection based on magnetic chitosan and silver/chitosan nanoparticles using surface-enhanced raman spectroscopy. Anal. Chem. 2015, 87, 11039–11047. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.T.; Liu, Y.Z.; Zhou, X.D.; Shen, A.G.; Hu, J.M. A turn-off sers-based detection platform for ultrasensitive detection of thrombin based on, enzymatic assays. Biosens. Bioelectron. 2013, 44, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Q.; Dai, Y.Y.; Jing, L.D.; Bai, J.; Liu, S.Z.; Zheng, K.G.; Pan, J. Subcellular localization of proline-rich tyrosine kinase 2 during oocyte fertilization and early-embryo development in mice. J. Reprod. Dev. 2016, 62, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ji, S.X.; Fang, X.L.; Wang, Q.G.; Li, Z.; Yao, F.Y.; Hou, L.; Dai, S.J. Protein kinase ltrpk1 influences cold adaptation and microtubule stability in rice. J. Plant Growth Regul. 2013, 32, 483–490. [Google Scholar] [CrossRef]
- Zhao, S.S.; Jiang, Y.X.; Zhao, Y.; Huang, S.J.; Yuan, M.; Zhao, Y.X.; Guo, Y. Casein kinase1-like protein2 regulates actin filament stability and stomatal closure via phosphorylation of actin depolymerizing factor. Plant Cell 2016, 28, 1422–1439. [Google Scholar] [CrossRef] [PubMed]
- Cottat, M.; Yasukuni, R.; Homma, Y.; Lidgi-Guigui, N.; Varin-Blank, N.; de la Chapelle, M.L.; Le Roy, C. Phosphorylation impact on spleen tyrosine kinase conformation by surface enhanced raman spectroscopy. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Kyaw, Y.M.E.; Tan, E.K.M.; Bekale, L.; Kang, M.W.C.; Kim, S.S.Y.; Tan, I.; Lam, K.P.; Kah, J.C.Y. Quantitative and label-free detection of protein kinase a activity based on surface-enhanced raman spectroscopy with gold nanostars. Anal. Chem. 2018, 90, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Xing, W.X.; Shan, S.J.; Zhang, S.Q.; Li, Y.Q.; Li, T.; An, L.; Yang, G.W. Characterization and immune response expression of the rig-i-like receptor mda5 in common carp cyprinus carpio. J. Fish Biol. 2016, 88, 2188–2202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Liu, D.Z.; Wang, L.; Li, T.; Chang, Q.; An, L.G.; Yang, G.W. Characterization of igm-binding protein: A pigr-like molecule expressed by intestinal epithelial cells in the common carp (cyprinus carpio l.). Vet. Immunol. Immunopathol. 2015, 167, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, H.; Peng, S.Q.; Zhang, F.M.; An, L.G.; Yang, G.W. Molecular characterization and expression pattern of x box-binding protein-1 (xbp1) in common carp (cyprinus carpio l.): Indications for a role of xbp1 in antibacterial and antiviral immunity. Fish Shellfish Immunol. 2017, 67, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Zou, S.S.; Zhai, L.J.; Wang, Y.; Zhang, F.M.; An, L.G.; Yang, G.W. Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol. 2017, 71, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Penn, M.A.; Drake, D.M.; Driskell, J.D. Accelerated surface-enhanced raman spectroscopy (sers)-based immunoassay on a gold-plated membrane. Anal. Chem. 2013, 85, 8609–8617. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.F.; Yan, Y.R.; Jiang, G.Q.; Adkins, J.; Shi, J.; Jiang, G.M.; Tian, S. Using a silver-enhanced microarray sandwich structure to improve sers sensitivity for protein detection. Anal. Bioanal. Chem. 2014, 406, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Harpster, M.H.; Zhang, H.; Mecham, J.O.; Wilson, W.C.; Johnson, P.A. A versatile sers-based immunoassay for immunoglobulin detection using antigen-coated gold nanoparticles and malachite green-conjugated protein a/g. Biosens. Bioelectron. 2010, 26, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Yang, G.W.; Pan, J.; Zhang, C. Tumor necrosis factor alpha knockout increases fertility of mice. Theriogenology 2011, 75, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.J.; Qi, C.C.; Zhu, Y.Y.; Li, H.; An, L.G.; Yang, G.W. Expression profile of carp ifn correlate with the up-regulation of interferon regulatory factor-1 (irf-1) in vivo and in vitro: The pivotal molecules in antiviral defense. Fish Shellfish Immunol. 2016, 52, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Winkler, K.; Kowalska, A.; Witkowska, E.; Szymborski, T.; Janeczek, A.; Waluk, J. Sers-based immunoassay in a microfluidic system for the multiplexed recognition of interleukins from blood plasma: Towards picogram detection. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Sprynskyy, M.; Winkler, K.; Szymborski, T. Ultrasensitive sers immunoassay based on diatom biosilica for detection of interleukins in blood plasma. Anal. Bioanal. Chem. 2017, 409, 6337–6347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, L.J.; Jiang, J.H. Surface-enhanced raman spectroscopy-based, homogeneous, multiplexed immunoassay with antibody-fragments-decorated gold nanoparticles. Anal. Chem. 2013, 85, 9213–9220. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, S.; Ma, L.; Li, F.L.; Zheng, Z.D.; Chai, R.F.; Hou, Y.H.; Xie, Y.B.; Li, G.R. Oogenesis, vitellogenin-mediated ovarian degeneration and immune response in the annual fish nothobranchius guentheri. Fish Shellfish Immunol. 2017, 66, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.Q.; Liang, X.J.; Liu, Q.Y.; Liang, A.H.; Jiang, Z.L. A novel nanocatalytic sers detection of trace human chorionic gonadotropin using labeled-free vitoria blue 4r as molecular probe. Biosens. Bioelectron. 2016, 85, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, L.; Cheng, D.; Liu, T.; An, L.G.; Li, W.P.; Zhang, C. Low-density lipoprotein receptor affects the fertility of female mice. Reprod. Fertil. Dev. 2015, 27, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.F.; Parchur, A.K.; Gilbertson, T.A.; Zhou, A.H. Sers-fluorescence bimodal nanoprobes for in vitro imaging of the fatty acid responsive receptor gpr120. Anal. Methods 2018, 10, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Kang, H.M.; Ko, E.; Jun, B.H.; Lee, H.Y.; Lee, Y.S.; Jeang, D.H. Psa detection with femtomolar sensitivity and a broad dynamic range using sers nanoprobes and an area-scanning method. ACS Sens. 2016, 1, 645–649. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, G.H.; Wei, F.D.; Zhang, A.X.; Yang, J.; Hu, Q. Detection of cea in human serum using surface-enhanced raman spectroscopy coupled with antibody-modified au and gamma-fe2o3@au nanoparticles. J. Pharm. Biomed. Anal. 2016, 121, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.D.; Dong, J.; Xie, W.; Tao, L.; Lu, W.B.; Wang, Y.; Qian, W.P. Sers tags-based novel monodispersed hollow gold nanospheres for highly sensitive immunoassay of cea. J. Mater. Sci. 2015, 50, 3329–3336. [Google Scholar] [CrossRef]
- Gao, R.K.; Cheng, Z.Y.; Demello, A.J.; Choo, J. Wash-free magnetic immunoassay of the psa cancer marker using sers and droplet microfluidics. Lab Chip 2016, 16, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.J.; Hu, Y.H.; Wei, H. Enzymatically activated reduction-caged sers reporters for versatile bioassays. Analyst 2017, 142, 2322–2326. [Google Scholar] [CrossRef] [PubMed]
- Song, G.F.; Zhou, H.; Gu, J.J.; Liu, Q.L.; Zhang, W.; Su, H.L.; Su, Y.S.; Yao, Q.H.; Zhang, D. Tumor marker detection using surface enhanced raman spectroscopy on 3d au butterfly wings. J. Mater. Chem. B 2017, 5, 1594–1600. [Google Scholar] [CrossRef]
- Karaballi, R.A.; Merchant, S.; Power, S.R.; Brosseau, C.L. Electrochemical surface-enhanced raman spectroscopy (ec-sers) study of the interaction between protein aggregates and biomimetic membranes. Phys. Chem. Chem. Phys. 2018, 20, 4513–4526. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.S.; Jones, S.; Pramanik, A.; Ray, P.C. Nanoarchitecture based sers for biomolecular fingerprinting and label-free disease markers diagnosis. Acc. Chem. Res. 2016, 49, 2725–2735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, P.; Mao, L.; Hou, Y.; Li, D. Determination of brain injury biomarkers by surface-enhanced raman scattering using hollow gold nanospheres. RSC Adv. 2018, 8, 3143–3150. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Li, H.; Mao, L.; Wang, Y.; Sun, B. Sers based protocol flow glass-hemostix for detection of neuron-specific enolase in blood plasma. New J. Chem. 2018. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, P.; Kasani, S.; Wu, S.; Yang, F.; Lewis, S.; Nayeem, S.; Engler-Chiurazzi, E.B.; Wigginton, J.G.; Simpkins, J.W.; et al. Paper-based surface-enhanced raman scattering lateral flow strip for detection of neuron-specific enolase in blood plasma. Anal. Chem. 2017, 89, 10104–10110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Ju, Z.H.; Huang, J.M.; Hou, M.H.; Zhou, L.; Qi, C.; Zhang, Y.; Gao, Q.; Pan, Q.; Li, G.R.; et al. The relationship between the variants of the bovine mbl2 gene and milk production traits, mastitis, serum mbl-c levels and complement activity. Vet. Immunol. Immunopathol. 2012, 148, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, F.M.; Guo, H.Y.; Zhu, Y.Y.; Yuan, J.D.; Yang, G.W.; An, L.G. Molecular characterization of hepcidin gene in common carp (cyprinus carpio l.) and its expression pattern responding to bacterial challenge. Fish Shellfish Immunol. 2013, 35, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.J.; Liu, D.Z.; Wang, L.; Zhu, Y.Y.; Zhang, F.M.; Li, T.; An, L.G.; Yang, G.W. Identification and expression analysis of irak1 gene in common carp cyprinus carpio l.: Indications for a role of antibacterial and antiviral immunity. J. Fish Boil. 2015, 87, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Girel, K.; Yantcevich, E.; Arzumanyan, G.; Doroshkevich, N.; Bandarenka, H. Detection of DNA molecules by sers spectroscopy with silvered porous silicon as an active substrate. Phys. Status Solidi A Appl. Mater. Sci. 2016, 213, 2911–2915. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.L.; Zhu, L.; Chen, X.H.; Ma, Z.C.; Zhang, X.L.; Wang, J.N.; Wang, W.; Liu, Y.Q.; Chen, Q.D.; et al. Direct laser scribing of agnps@rgo biochip as a reusable sers sensor for DNA detection. Sens. Actuators B Chem. 2018, 270, 500–507. [Google Scholar] [CrossRef]
- Qian, Y.; Fan, T.T.; Yao, Y.; Shi, X.; Liao, X.J.; Zhou, F.Y.; Gao, F.L. Label-free and raman dyes-free surface-enhanced raman spectroscopy for detection of DNA. Sens. Actuators B Chem. 2018, 254, 483–489. [Google Scholar] [CrossRef]
- Yu, J.; Jeon, J.; Choi, N.; Lee, J.O.; Kim, Y.P.; Choo, J. Sers-based genetic assay for amplification-free detection of prostate cancer specific pca3 mimic DNA. Sens. Actuators B Chem. 2017, 251, 302–309. [Google Scholar] [CrossRef]
- Wang, R.; Kim, K.; Choi, N.; Wang, X.; Lee, J.; Jeon, J.H.; Rhie, G.-E.; Choo, J. Highly sensitive detection of high-risk bacterial pathogens using sers-based lateral flow assay strips. Sens. Actuators B Chem. 2018, 270, 72–79. [Google Scholar] [CrossRef]
- Ye, S.J.; Wu, Y.Y.; Zhang, W.; Li, N.; Tang, B. A sensitive sers assay for detecting proteins and nucleic acids using a triple-helix molecular switch for cascade signal amplification. Chem. Commun. 2014, 50, 9409–9412. [Google Scholar] [CrossRef] [PubMed]
- Kammer, E.; Olschewski, K.; Bocklitz, T.; Rosch, P.; Weber, K.; Cialla, D.; Popp, J. A new calibration concept for a reproducible quantitative detection based on sers measurements in a microfluidic device demonstrated on the model analyte adenine. Phys. Chem. Chem. Phys. 2014, 16, 9056–9063. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.L.; Zheng, J.; Tan, Y.J.; Tan, G.X.; Li, J.S.; Li, Y.H.; Li, X.; Zhou, Z.G.; Yang, R.H. Ultrasensitive detection of single nucleotide polymorphism in human mitochondrial DNA utilizing ion-mediated cascade surface-enhanced raman spectroscopy amplification. Anal. Chem. 2015, 87, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.W.; Bao, M.; Shan, Y.B.; Li, W.; Shi, H.C. Rapid detection and identification of mirnas by surface-enhanced raman spectroscopy using hollow au nanoflowers substrates. J. Nanomater. 2017. [Google Scholar] [CrossRef]
- Driskell, J.D.; Tripp, R.A. Label-free sers detection of microrna based on affinity for an unmodified silver nanorod array substrate. Chem. Commun. 2010, 46, 3298–3300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Bai, J.H.; Zhou, Q.F.; Li, J.S.; Li, Y.H.; Yang, J.F.; Yang, R.H. DNA-templated in situ growth of agnps on swnts: A new approach for highly sensitive sers assay of microrna. Chem. Commun. 2015, 51, 6552–6555. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wu, Y.; Zhai, X.; Tang, B. Asymmetric signal amplification for simultaneous sers detection of multiple cancer markers with significantly different levels. Anal. Chem. 2015, 87, 8242–8249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tian, Y.F.; Yin, B.C.; Ye, B.C. Simultaneous surface-enhanced raman spectroscopy detection of multiplexed microrna biomarkers. Anal. Chem. 2017, 89, 6121–6129. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Choi, J.; Kim, Y.; Lee, Y.; Kim, J.; Lee, S.; Chung, H. Feasibility study for combination of field-flow fractionation (fff)-based separation of size-coded particle probes with amplified surface enhanced raman scattering (sers) tagging for simultaneous detection of multiple mirnas. J. Chromatogr. A 2018, 1556, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Xu, C.X.; Lu, J.F.; Zhu, Z.; Zhu, Q.X.; Manohari, A.G.; Shi, Z.L. Template-free synthesis of porous zno/ag microspheres as recyclable and ultra-sensitive sers substrates. Appl. Surf. Sci. 2018, 427, 830–836. [Google Scholar] [CrossRef]
- Sooraj, K.P.; Ranjan, M.; Rao, R.; Mukherjee, S. Sers based detection of glucose with lower concentration than blood glucose level using plasmonic nanoparticle arrays. Appl. Surf. Sci. 2018, 447, 576–581. [Google Scholar] [CrossRef]
- Qi, G.H.; Jia, K.Q.; Fu, C.C.; Xu, S.P.; Xu, W.Q. A highly sensitive sers sensor for quantitative analysis of glucose based on the chemical etching of silver nanoparticles. J. Opt. 2015, 17. [Google Scholar] [CrossRef]
- Fu, C.; Jin, S.; Oh, J.; Xu, S.P.; Jung, Y.M. Facile detection of glucose in human serum employing silver-ion-guided surface-enhanced raman spectroscopy signal amplification. Analyst 2017, 142, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.J.; Li, S.; Han, F.; Liu, L.Q.; Xu, L.G.; Ma, W.; Kuang, H.; Li, A.K.; Wang, L.B.; Xu, C.L. Sers-active au@ag nanorod dimers for ultrasensitive dopamine detection. Biosens. Bioelectron. 2015, 71, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; De Bleye, C.; Cailletaud, J.; Sacre, P.Y.; Van Lerberghe, P.B.; Rogister, B.; Rance, G.A.; Aylott, J.W.; Hubert, P.; Ziemons, E. Development of a sers strategy to overcome the nanoparticle stabilisation effect in serum-containing samples: Application to the quantification of dopamine in the culture medium of pc-12 cells. Talanta 2018, 186, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.M.; Qin, M.; Li, P.; Zhou, B.B.; Tang, X.H.; Ge, M.H.; Yang, L.B.; Liu, J.H. Probing catecholamine neurotransmitters based on iron-coordination surface-enhanced resonance raman spectroscopy label. Sens. Actuators B Chem. 2018, 268, 350–358. [Google Scholar] [CrossRef]
- Moody, A.S.; Sharma, B. Multi-metal, multi-wavelength surface-enhanced raman spectroscopy detection of neurotransmitters. ACS Chem. Neurosci. 2018, 9, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernandez, I.; Afseth, N.K.; Lopez-Luke, T.; Contreras-Torres, F.F.; Wold, J.P.; Ornelas-Soto, N. Surface enhanced raman spectroscopy of phenolic antioxidants: A systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vib. Spectrosc. 2017, 89, 113–122. [Google Scholar] [CrossRef]
- Li, Y.; Driver, M.; Decker, E.; He, L.L. Lipid and lipid oxidation analysis using surface enhanced raman spectroscopy (sers) coupled with silver dendrites. Food Res. Int. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Tang, G.Y.; Xu, P.L.; Ma, W.H.; Wang, F.; Liu, Z.J.; Wan, S.B.; Shan, L. Seed-specific expression of atlec1 increased oil content and altered fatty acid composition in seeds of peanut (arachis hypogaea l.). Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Li, M.; Li, K.; Song, J.; Wang, B.S. Increase in unsaturated fatty acids in membrane lipids of suaeda salsa l. Enhances protection of photosystem ii under high salinity. Photosynthetica 2010, 48, 623–629. [Google Scholar] [CrossRef]
- Sun, Y.L.; Li, F.; Su, N.; Sun, X.L.; Zhao, S.J.; Meng, Q.W. The increase in unsaturation of fatty acids of phosphatidylglycerol in thylakoid membrane enhanced salt tolerance in tomato. Photosynthetica 2010, 48, 400–408. [Google Scholar] [CrossRef]
- Sui, N.; Han, G.L. Salt-induced photoinhibition of psii is alleviated in halophyte thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol. Plant. 2014, 36, 983–992. [Google Scholar] [CrossRef]
- Radu, A.I.; Ryabchykov, O.; Bocklitz, T.W.; Huebner, U.; Weber, K.; Cialla-May, D.; Popp, J. Toward food analytics: Fast estimation of lycopene and beta-carotene content in tomatoes based on surface enhanced raman spectroscopy (sers). Analyst 2016, 141, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.Y.; Chen, H.Y.; Ling, Y.C.; Huang, W.S.; Hung, Y.C.; Gwo, S.; Ho, R.M. A polymer-based sers-active substrate with gyroid-structured gold multibranches. J. Mater. Chem. C 2014, 2, 4667–4675. [Google Scholar] [CrossRef]
- Meng, X.; Yang, D.Y.; Li, X.D.; Zhao, S.Y.; Sui, N.; Meng, Q.W. Physiological changes in fruit ripening caused by overexpression of tomato slan2, an r2r3-myb factor. Plant Physiol. Biochem. 2015, 89, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.; Liu, T.Y.; Wang, K.S.; Tsai, K.T.; Chen, Z.X.; Chang, Y.C.; Tseng, Y.Q.; Wang, C.H.; Wang, J.K.; Wang, Y.L. Sers detection of biomolecules by highly sensitive and reproducible raman-enhancing nanoparticle array. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Wang, W.Q.; Li, M.; Wan, S.B.; Sui, N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol. Plant. 2017, 39. [Google Scholar] [CrossRef]
- Wrona, M.; Salafranca, J.; Rocchia, M.; Nerin, C. Application of sers to the determination of butylated hydroxyanisole in edible and essential oils. Spectroscopy 2015, 30, 40–45. [Google Scholar]
- Huang, C.C.; Chen, W.L. A sers method with attomolar sensitivity: A case study with the flavonoid catechin. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, Y.J.; Gao, J.W.; Ma, C.L.; Bi, Y.P. A comparative transcriptome analysis of a wild purple potato and its red mutant provides insight into the mechanism of anthocyanin transformation. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, F.; Zhou, J.J.; Chen, F.; Wang, B.S.; Xie, X.Z. Phytochrome b control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol.Biol. 2012, 78, 289–300. [Google Scholar] [CrossRef] [PubMed]
- He, Y.A.; Li, Y.P.; Cui, L.X.; Xie, L.X.; Zheng, C.K.; Zhou, G.H.; Zhou, J.J.; Xie, X.Z. Phytochrome b negatively affects cold tolerance by regulating osdreb1 gene expression through phytochrome interacting factor-like protein ospil16 in rice. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Z.; Sun, H.Z.; Chen, M.; Wang, B.S. Light-regulated betacyanin accumulation in euhalophyte suaeda salsa calli. Plant Cell Tissue Organ Cult. 2010, 102, 99–107. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Sun, H.Z.; Gao, Y.; Sui, N.; Wang, B.S. Growth regulator-induced betacyanin accumulation and dopa-4,5-dioxygenase (doda) gene expression in euhalophyte suaeda salsa calli. In Vitro Cell. Dev. Biol. Plant 2011, 47, 391–398. [Google Scholar] [CrossRef]
- Zaffino, C.; Russo, B.; Bruni, S. Surface-enhanced raman scattering (sers) study of anthocyanidins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zaffino, C.; Bruni, S.; Russo, B.; Pilu, R.; Lago, C.; Colonna, G.M. Identification of anthocyanins in plant sources and textiles by surface-enhanced raman spectroscopy (sers). J. Raman Spectrosc. 2016, 47, 269–276. [Google Scholar] [CrossRef]
- De Luca, E.; Redaelli, M.; Zaffino, C.; Bruni, S. A sers and hplc study of traditional dyes from native Chinese plants. Vib. Spectrosc. 2018, 95, 62–67. [Google Scholar] [CrossRef]
- Gu, X.L.; Jin, Y.; Dong, F.; Cai, Y.Q.; You, Z.G.; You, J.H.; Zhang, L.Y.; Du, S.H. Toward rapid analysis, forecast and discovery of bioactive compounds from herbs by jointly using thin layer chromatography and ratiometric surface-enhanced raman spectroscopy technique. J. Pharm. Biomed. Anal. 2018, 153, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.P.; Bai, X.Y.; Zhao, B. Based on sers conformational studies of ginsenoside rb1 and its metabolites before and after combined with human serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.K.; Fang, X.; Cao, Y.; Xiao, H.; He, L.L. Monitoring the chemical production of citrus-derived bioactive 5-demethylnobiletin using surface-enhanced raman spectroscopy. J. Agric. Food Chem. 2013, 61, 8079–8083. [Google Scholar] [CrossRef] [PubMed]
- Buyukgoz, G.G.; Soforoglu, M.; Akgul, N.B.; Boyaci, I.H. Spectroscopic fingerprint of tea varieties by surface enhanced raman spectroscopy. J. Food Sci. Technol. Mysore 2016, 53, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Leavitt, S.D.; Zhao, Z.T.; Zhang, L.L.; Arup, U.; Grube, M.; Perez-Ortega, S.; Printzen, C.; Sliwa, L.; Kraichak, E.; et al. Towards a revised generic classification of lecanoroid lichens (lecanoraceae, ascomycota) based on molecular, morphological and chemical evidence. Fungal Divers. 2016, 78, 293–304. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.Y.; Biener, G.; Xiong, E.H.; Malik, S.; Eaton, N.; Zhao, C.Z.; Raicu, V.; Kong, H.Z.; Zhao, D.Z. Carbonic anhydrases function in anther cell differentiation downstream of the receptor-like kinase ems1. Plant Cell 2017, 29, 1335–1356. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.P.; Han, R.; Li, H.S.; Song, J.M.; Yan, H.F.; Li, G.Y.; Liu, A.F.; Cao, X.Y.; Guo, J.; Zhai, S.N.; et al. Agronomic traits and molecular marker identification of wheat-aegilops caudata addition lines. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Seifert, S.; Merk, V.; Kneipp, J. Identification of aqueous pollen extracts using surface enhanced raman scattering (sers) and pattern recognition methods. J. Biophotonics 2016, 9, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.I.; Kuellmer, M.; Giese, B.; Huebner, U.; Weber, K.; Cialla-May, D.; Popp, J. Surface-enhanced raman spectroscopy (sers) in food analytics: Detection of vitamins b-2 and b-12 in cereals. Talanta 2016, 160, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Z.; Qiu, J.J.; Agarwal, G.; Wang, J.S.; Ren, X.Z.; Xia, H.; Guo, B.Z.; Ma, C.L.; Wan, S.B.; Bertioli, D.J.; et al. Genome-wide discovery of microsatellite markers from diploid progenitor species, arachis duranensis and a. Ipaensis, and their application in cultivated peanut (a. Hypogaea). Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Zhang, Q.; Cui, F.; Hou, L.; Zhao, S.Z.; Xia, H.; Qiu, J.J.; Li, T.T.; Zhang, Y.; Wang, X.J.; et al. Genome-wide analysis of gene expression provides new insights into cold responses in thellungiella salsuginea. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Wei, X.C.; Zhao, X.; Wang, B.S.; Sui, N. Transcription profiles of genes related to hormonal regulations under salt stress in sweet sorghum. Plant Mol. Biol. Rep. 2017, 35, 586–599. [Google Scholar] [CrossRef]
- Muntean, C.M.; Leopold, N.; Halmagyi, A.; Valimareanu, S. Surface-enhanced raman spectroscopy of DNA from leaves of in vitro grown apple plants. J. Raman Spectrosc. 2011, 42, 844–850. [Google Scholar] [CrossRef]
- Muntean, C.M.; Bratu, I.; Leopold, N.; Purcaru, M.A.P. Subpicosecond dynamics in DNA from leaves of in vitro-grown apple plants: A sers study. Spectrosc. -Biomed. Appl. 2011, 26, 59–68. [Google Scholar] [CrossRef]
- Muntean, C.M.; Leopold, N.; Tripon, C.; Coste, A.; Halmagyi, A. Surface-enhanced raman spectroscopy of genomic DNA from in vitro grown tomato (lycopersicon esculentum mill.) cultivars before and after plant cryopreservation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Portaccio, M.; Tate, R.; Lepore, M.; Delfino, I. Fructose and pectin detection in fruit-based food products by surface-enhanced raman spectroscopy. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Shao, F.X.; Xu, P.L.; Shan, L.; Liu, Z.J. Overexpression of a peanut nac gene, ahnac4, confers enhanced drought tolerance in tobacco. Russ. J. Plant Physiol. 2017, 64, 525–535. [Google Scholar] [CrossRef]
- An, J.; Hou, L.; Li, C.; Wang, C.X.; Xia, H.; Zhao, C.Z.; Li, C.S.; Zheng, Y.X.; Zhao, Y.X.; Wang, X. Cloning and expression analysis of four della genes in peanut. Russ. J. Plant Physiol. 2015, 62, 116–126. [Google Scholar] [CrossRef]
- Gezer, R.G.; Liu, G.L.; Kokini, J.L. Development of a biodegradable. Sensor platform from gold coated zein nanophotonic films to detect peanut allergen, ara h1, using surface enhanced raman spectroscopy. Talanta 2016, 150, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Palanco, M.E.; Mogensen, K.B.; Kneipp, K. Raman spectroscopic probing of plant material using sers. J. Raman Spectrosc. 2016, 47, 156–161. [Google Scholar] [CrossRef]
- Shen, A.G.; Guo, J.Z.; Xie, W.; Sun, M.X.; Richards, R.; Hu, J.M. Surface-enhanced raman spectroscopy in living plant using triplex Au-Ag-C core-shell nanoparticles. J. Raman Spectrosc. 2011, 42, 879–884. [Google Scholar] [CrossRef]
- Cepeda-Perez, E.; Aguilar-Hernandez, I.; Lopez-Luke, T.; Piazza, V.; Carriles, R.; Ornelas-Soto, N.; de la Rosa, E. Interaction of tga@cdte quantum dots with an extracellular matrix of haematococcus pluvialis microalgae detected using surface-enhanced raman spectroscopy (sers). Appl. Spectrosc. 2016, 70, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Huang, F.; Mason, D.; Zhao, G.Y.; Johnson, G.N.; Mullineaux, C.W.; Liu, L.N. Dissecting the native architecture and dynamics of cyanobacterial photosynthetic machinery. Mol. Plant. 2017, 10, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Qi, C.C.; Shan, S.J.; Zhang, F.M.; Li, H.; An, L.G.; Yang, G.W. Characterization of common carp (cyprinus carpio l.) interferon regulatory factor 5 (irf5) and its expression in response to viral and bacterial challenges. BMC Vet. Res. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
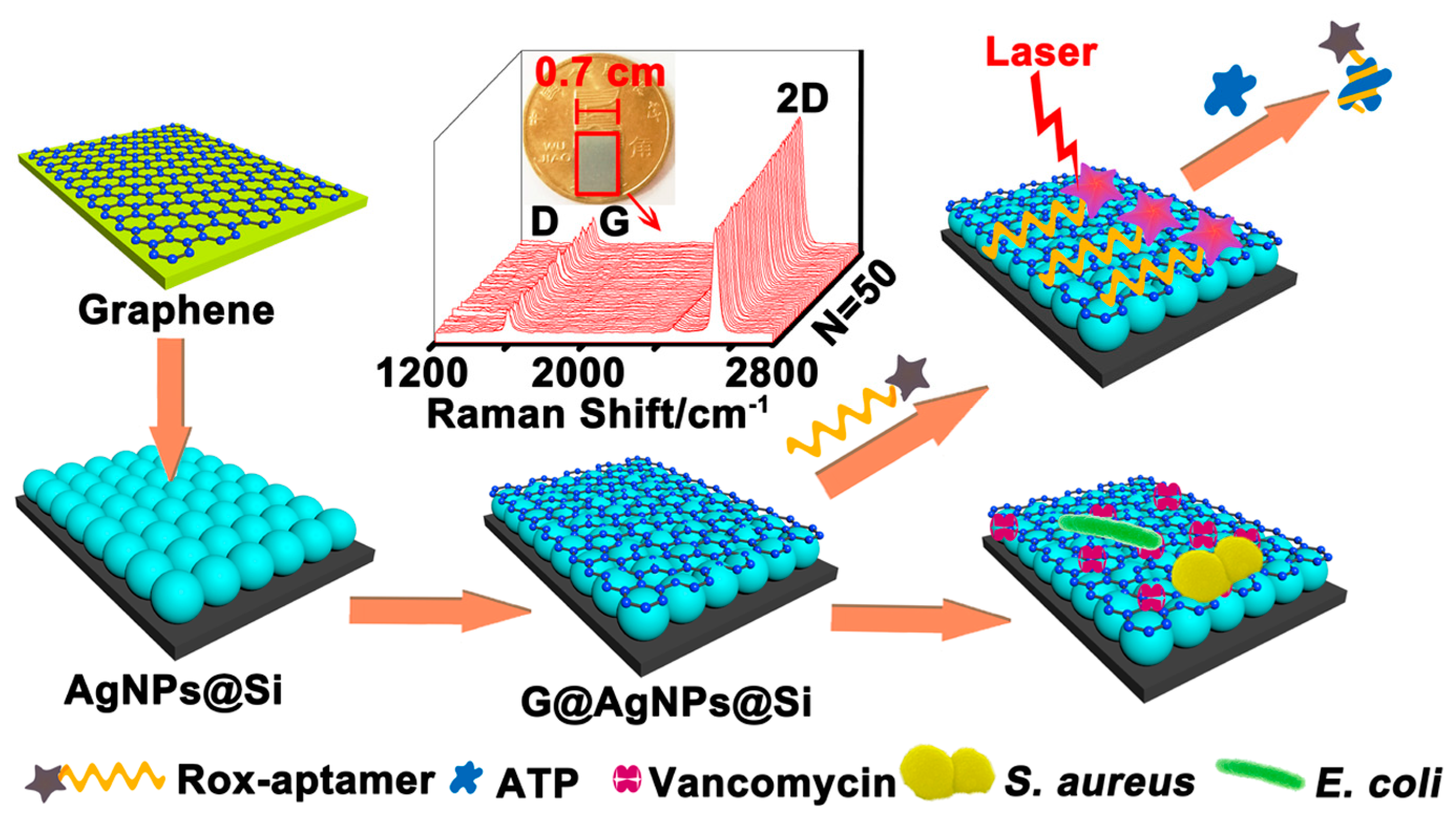
- Meng, X.Y.; Wang, H.Y.; Chen, N.; Ding, P.; Shi, H.Y.; Zhai, X.; Su, Y.Y.; He, Y. A graphene-silver nanoparticle-silicon sandwich sers chip for quantitative detection of molecules and capture, discrimination, and inactivation of bacteria. Anal. Chem. 2018, 90, 5646–5653. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.C.; Li, B.; Yao, R.Z.; Li, Z.P.; Wang, X.W.; Dong, X.L.; Qu, H.; Li, Q.X.; Li, N.; Chi, H.; et al. Intuitive label-free sers detection of bacteria using aptamer-based in situ silver nanoparticles synthesis. Anal. Chem. 2017, 89, 9836–9842. [Google Scholar] [CrossRef] [PubMed]
- Efrima, S.; Zeiri, L. Understanding sers of bacteria. J. Raman Spectrosc. 2009, 40, 277–288. [Google Scholar] [CrossRef]
- Chauvet, R.; Lagarde, F.; Charrier, T.; Assaf, A.; Thouand, G.; Daniel, P. Microbiological identification by surface-enhanced raman spectroscopy. Appl. Spectrosc. Rev. 2017, 52, 123–144. [Google Scholar] [CrossRef]
- Shan, S.J.; Liu, D.Z.; Liu, R.R.; Zhu, Y.Y.; Li, T.; Zhang, F.M.; An, L.G.; Yang, G.W.; Li, H. Non-mammalian toll-like receptor 18 (tlr18) recognizes bacterial pathogens in common carp (cyprinus carpio l.): Indications for a role of participation in the nf-kappa b signaling pathway. Fish Shellfish Immunol. 2018, 72, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Lu, X.N. Development of a loop mediated isothermal amplification (lamp)—surface enhanced raman spectroscopy (sers) assay for the detection of salmonella enterica serotype enteritidis. Theranostics 2016, 6, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Gracie, K.; Correa, E.; Mabbott, S.; Dougan, J.A.; Graham, D.; Goodacre, R.; Faulds, K. Simultaneous detection and quantification of three bacterial meningitis pathogens by sers. Chem. Sci. 2014, 5, 1030–1040. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.L.; Gong, N.C.; Jiang, X.; Huan, S.Y. Surface enhanced raman spectroscopy sensor based on magnetic beads-induced nanoparticles aggregation for detection of bacterial deoxyribonucleic acid. Chin. J. Anal. Chem. 2015, 43, 1676–1681. [Google Scholar] [CrossRef]
- Wang, Y.; Rauf, S.; Grewal, Y.S.; Spadafora, L.J.; Shiddiky, M.J.A.; Cangelosi, G.A.; Schlucker, S.; Trau, M. Duplex microfluidic sers detection of pathogen antigens with nanoyeast single-chain variable fragments. Anal. Chem. 2014, 86, 9930–9938. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.K.; Iavarone, A.T.; Coates, J.D. Surfaceomics and surface-enhanced raman spectroscopy of environmental microbes: Matching cofactors with redox-active surface proteins. Proteomics 2013, 13, 2761–2765. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Yang, J.K.; Noh, M.S.; Jo, A.; Jeong, S.; Lee, M.; Lee, S.; Chang, H.; Lee, H.; Jeon, S.J.; et al. One-step synthesis of silver nanoshells with bumps for highly sensitive near-ir sers nanoprobes. J. Mater. Chem. B 2014, 2, 4415–4421. [Google Scholar] [CrossRef]
- Xu, J.J.; Turner, J.W.; Idso, M.; Biryukov, S.V.; Rognstad, L.; Gong, H.; Trainer, V.L.; Wells, M.L.; Strom, M.S.; Yu, Q.M. In situ strain-level detection and identification of vibrio parahaemolyticus using surface-enhanced raman spectroscopy. Anal. Chem. 2013, 85, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Stephen, K.E.; Homrighausen, D.; DePalma, G.; Nakatsu, C.H.; Irudayaraj, J. Surface enhanced raman spectroscopy (sers) for the discrimination of arthrobacter strains based on variations in cell surface composition. Analyst 2012, 137, 4280–4286. [Google Scholar] [CrossRef] [PubMed]
- Muhlig, A.; Bocklitz, T.; Labugger, I.; Dees, S.; Henk, S.; Richter, E.; Andres, S.; Merker, M.; Stockel, S.; Weber, K.; et al. Loc-sers: A promising closed system for the identification of mycobacteria. Anal. Chem. 2016, 88, 7998–8004. [Google Scholar] [CrossRef] [PubMed]
- DeJong, C.S.; Wang, D.I.; Polyakov, A.; Rogacs, A.; Simske, S.J.; Shkolnikov, V. Bacterial detection and differentiation via direct volatile organic compound sensing with surface enhanced raman spectroscopy. Chemistryselect 2017, 2, 8431–8435. [Google Scholar] [CrossRef]
- Guo, J.X.; Liu, Y.; Chen, Y.L.; Li, J.Q.; Ju, H.X. A multifunctional sers sticky note for real- time quorum sensing tracing and inactivation of bacterial biofilms. Chem. Sci. 2018, 9, 5906–5911. [Google Scholar] [CrossRef] [PubMed]
- Premasiri, W.R.; Lee, J.C.; Sauer-Budge, A.; Theberge, R.; Costello, C.E.; Ziegler, L.D. The biochemical origins of the surface-enhanced raman spectra of bacteria: A metabolomics profiling by sers. Anal. Bioanal. Chem. 2016, 408, 4631–4647. [Google Scholar] [CrossRef] [PubMed]
- Zukovskaja, O.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Detection of pseudomonas aeruginosa metabolite pyocyanin in water and saliva by employing the sers technique. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, H.J.; He, H.B.; Wang, C.F.; Gao, Y.D.; Zhong, Q.F.; Wang, X.H.; Zeng, Y.J. Study on the hemolysin phenotype and the genetype distribution of staphyloccocus aureus caused bovine mastitis in shandong dairy farms. Int. J. Appl. Res. Vet. Med. 2011, 9, 416–421. [Google Scholar]
- Wang, X.G.; Huang, J.M.; Feng, M.Y.; Ju, Z.H.; Wang, C.F.; Yang, G.W.; Yuan, J.D.; Zhong, J.F. Regulatory mutations in the a2m gene are involved in the mastitis susceptibility in dairy cows. Anim. Genet. 2014, 45, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Han, X.M.; Wang, Z.M.; Yang, Z.; Zhang, W.M.; Li, J.; Yang, H.H.; Ling, X.Y.; Xing, B.G. A live bacteria sers platform for the in situ monitoring of nitric oxide release from a single mrsa. Chem. Commun. 2018, 54, 7022–7025. [Google Scholar] [CrossRef] [PubMed]
- Pekdemir, M.E.; Erturkan, D.; Kulah, H.; Boyaci, I.H.; Ozgen, C.; Tamer, U. Ultrasensitive and selective homogeneous sandwich immunoassay detection by surface enhanced raman scattering (sers). Analyst 2012, 137, 4834–4840. [Google Scholar] [CrossRef] [PubMed]
- He, C.Q.; Liu, Y.X.; Wang, H.M.; Hou, P.L.; He, H.B.; Ding, N.Z. New genetic mechanism, origin and population dynamic of bovine ephemeral fever virus. Vet. Microbiol. 2016, 182, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.L.; Wang, H.M.; Zhao, G.M.; He, C.Q.; He, H.B. Rapid detection of infectious bovine rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet. Res. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.W.; Tian, F.L.; Lan, Z.R.; Huang, B.; Zhuang, W.Z. Selection characterization on overlapping reading frame of multiple-protein-encoding p gene in newcastle disease virus. Vet. Microbiol. 2010, 144, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.L.; Zhao, G.M.; He, C.Q.; Wang, H.M.; He, H.B. Biopanning of polypeptides binding to bovine ephemeral fever virus g(1) protein from phage display peptide library. BMC Vet. Res. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, J.Q.; Zhang, Y.Y.; Guo, L.H.; Cong, X.Y.; Du, Y.J.; Li, J.; Sun, W.B.; Shi, J.L.; Peng, J.; et al. Concurrent highly pathogenic porcine reproductive and respiratory syndrome virus infection accelerates haemophilus parasuis infection in conventional pigs. Vet. Microbiol. 2012, 158, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Nam, J.S.; Yang, S.E.; Shin, H.; Jang, Y.H.; Bae, G.U.; Kang, T.; Lim, K.I.; Choi, Y. Identification of newly emerging influenza viruses by surface-enhanced raman spectroscopy. Anal. Chem. 2015, 87, 11652–11659. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Witkowska, E.; Winkler, K.; Dziecielewski, I.; Weyher, J.L.; Waluk, J. Detection of hepatitis b virus antigen from human blood: Sers immunoassay in a microfluidic system. Biosens. Bioelectron. 2015, 66, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, L.; Zhang, F.D.; Song, Z.G.; Hu, Y.W.; Ji, Y.J.; Shen, J.Y.; Li, B.; Lu, H.Z.; Yang, H.F. A promising magnetic sers immunosensor for sensitive detection of avian influenza virus. Biosens. Bioelectron. 2017, 89, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shi, J.; Wu, X.; Peng, Z.; Xin, C.; Zhang, L.; Liu, Y.; Gao, M.; Xu, S.; Han, H.; et al. Presence of torque teno sus virus 1 and 2 in porcine circovirus 3-positive pigs. Transbound. Emerg. Dis. 2018, 65, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wu, X.; Zhang, L.; Xin, C.; Liu, Y.; Shi, J.; Peng, Z.; Xu, S.; Fu, F.; Yu, J.; et al. The occurrence of porcine circovirus 3 without clinical infection signs in shandong province. Transbound. Emerg. Dis. 2017, 64, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.H.; Li, W.T.; Lu, D.L.; Chen, K.; He, Q.G.; Han, H.Y.; Zou, M.Q. A sers-based immunoassay for porcine circovirus type 2 using multi-branched gold nanoparticles. Microchim. Acta 2013, 180, 1501–1507. [Google Scholar] [CrossRef]
- Luo, Z.H.; Chen, L.N.; Liang, C.J.; Wei, Q.M.; Chen, Y.; Wang, J. Porous carbon films decorated with silver nanoparticles as a sensitive sers substrate, and their application to virus identification. Microchim. Acta 2017, 184, 3505–3511. [Google Scholar] [CrossRef]
- Reyes, M.; Piotrowski, M.; Ang, S.K.; Chan, J.Q.; He, S.A.; Chu, J.J.H.; Kah, J.C.Y. Exploiting the anti-aggregation of gold nanostars for rapid detection of hand, foot, and mouth disease causing enterovirus 71 using surface-enhanced raman spectroscopy. Anal. Chem. 2017, 89, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Purra, M.; Carre-Camps, M.; de Puig, H.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Surface-enhanced raman spectroscopy-based sandwich immunoassays for multiplexed detection of zika and dengue viral biomarkers. ACS Infect. Dis. 2017, 3, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Gong, T.X.; Hong, Z.Y.; Qiu, S.F.; Pan, J.J.; Tseng, C.Y.; Feng, S.Y.; Chen, R.; Kong, K.V. Metal carbonyls for the biointerference-free ratiometric surface-enhanced raman spectroscopy-based assay for cell-free circulating DNA of epstein-barr virus in blood. Anal. Chem. 2018, 90, 7139–7147. [Google Scholar] [CrossRef] [PubMed]
- Harpster, M.H.; Zhang, H.; Sankara-Warrier, A.K.; Ray, B.H.; Ward, T.R.; Kollmar, J.P.; Carron, K.T.; Mecham, J.O.; Corcoran, R.C.; Wilson, W.C.; et al. Sers detection of indirect viral DNA capture using colloidal gold and methylene blue as a raman label. Biosens. Bioelectron. 2009, 25, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Malvadkar, N.A.; Demirel, G.; Poss, M.; Javed, A.; Dressick, W.J.; Demirel, M.C. Fabrication and use of electroless plated polymer surface-enhanced raman spectroscopy substrates for viral gene detection. J. Phys. Chem. C 2010, 114, 10730–10738. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wang, X.J.; Zhang, F.; Huo, X.B.; Fu, R.S.; Liu, J.J.; Sun, W.B.; Kang, D.M.; Jing, X. The identification of a bacterial strain bgi-1 isolated from the intestinal flora of blattella germanica, and its anti-entomopathogenic fungi activity. J. Econ. Entomol. 2013, 106, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Dina, N.E.; Gherman, A.M.R.; Chis, V.; Sarbu, C.; Wieser, A.; Bauer, D.; Haisch, C. Characterization of clinically relevant fungi via sers fingerprinting assisted by novel chemometric models. Anal. Chem. 2018, 90, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, E.; Jagielski, T.; Kaminska, A.; Kowalska, A.; Hryncewicz-Gwozdz, A.; Waluk, J. Detection and identification of human fungal pathogens using surface-enhanced raman spectroscopy and principal component analysis. Anal. Methods 2016, 8, 8427–8434. [Google Scholar] [CrossRef]
- Zivanovic, V.; Semini, G.; Laue, M.; Drescher, D.; Aebischer, T.; Kneipp, J. Chemical mapping of leishmania infection in live cells by sers microscopy. Anal. Chem. 2018, 90, 8154–8161. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.C.; Hennigan, S.L.; Henderson, K.C.; Clark, H.J.; Dluhy, R.A. Variable selection and biomarker correlation in the analysis of mycoplasma pneumoniae strains by surface-enhanced raman spectroscopy. Anal. Lett. 2017, 50, 2412–2425. [Google Scholar] [CrossRef]
- Garrett, N.L.; Sekine, R.; Dixon, M.W.A.; Tilley, L.; Bambery, K.R.; Wood, B.R. Bio-sensing with butterfly wings: Naturally occurring nano-structures for sers-based malaria parasite detection. Phys. Chem. Chem. Phys. 2015, 17, 21164–21168. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Li, S.; Zang, L.; Lu, X.; Zhang, H. Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 730. https://doi.org/10.3390/nano8090730
Jia M, Li S, Zang L, Lu X, Zhang H. Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy. Nanomaterials. 2018; 8(9):730. https://doi.org/10.3390/nano8090730
Chicago/Turabian StyleJia, Min, Shenmiao Li, Liguo Zang, Xiaonan Lu, and Hongyan Zhang. 2018. "Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy" Nanomaterials 8, no. 9: 730. https://doi.org/10.3390/nano8090730
APA StyleJia, M., Li, S., Zang, L., Lu, X., & Zhang, H. (2018). Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy. Nanomaterials, 8(9), 730. https://doi.org/10.3390/nano8090730