Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study
Abstract
:1. Introduction
2. Experimental Design, Materials, and Methods
2.1. Reagents and Chemicals
2.2. Synthesis of Metal-Organic Frameworks (MOFs)
2.3. Experimental Design
3. Results and Discussion
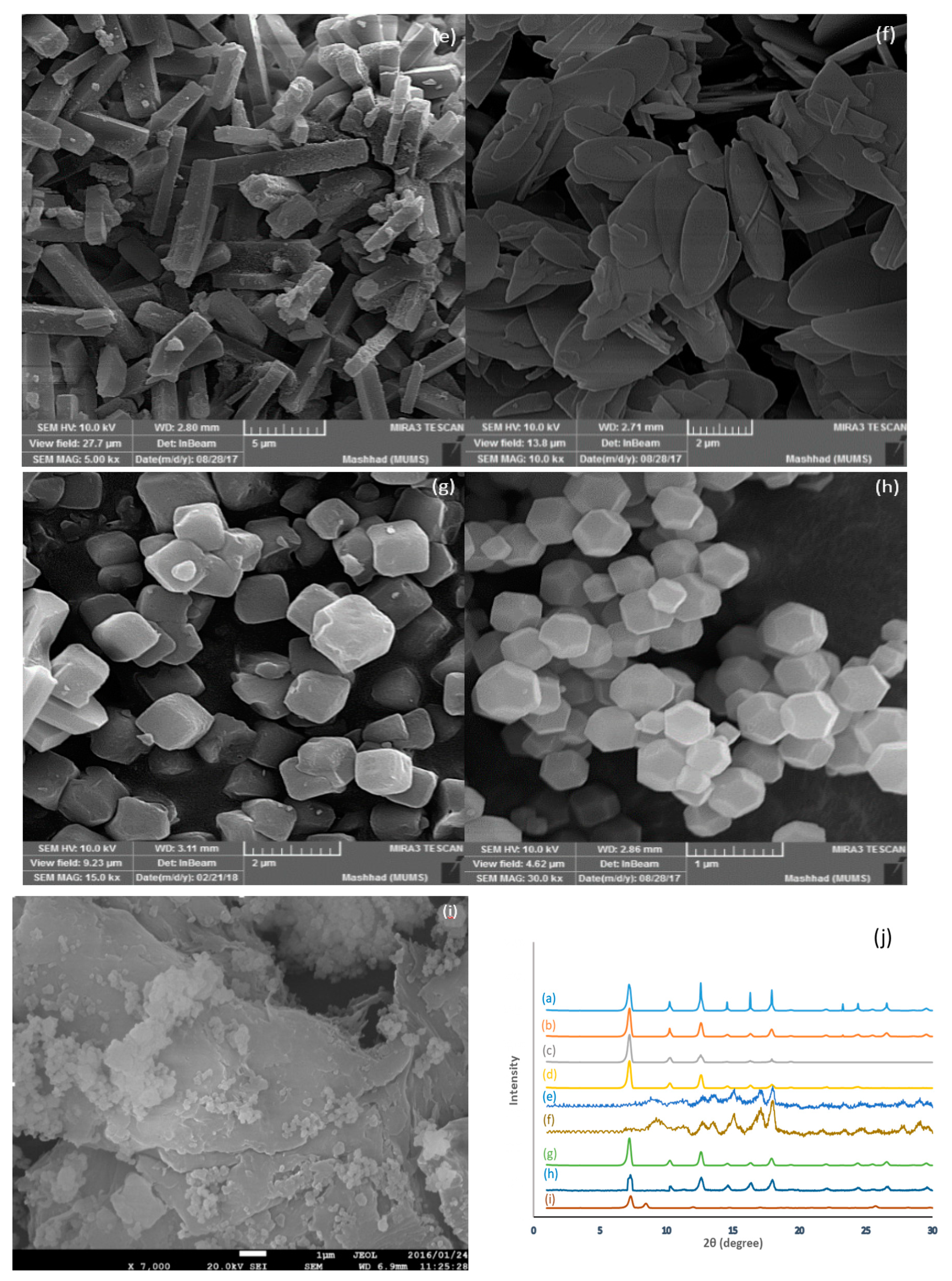
3.1. Adsorbent Characterization
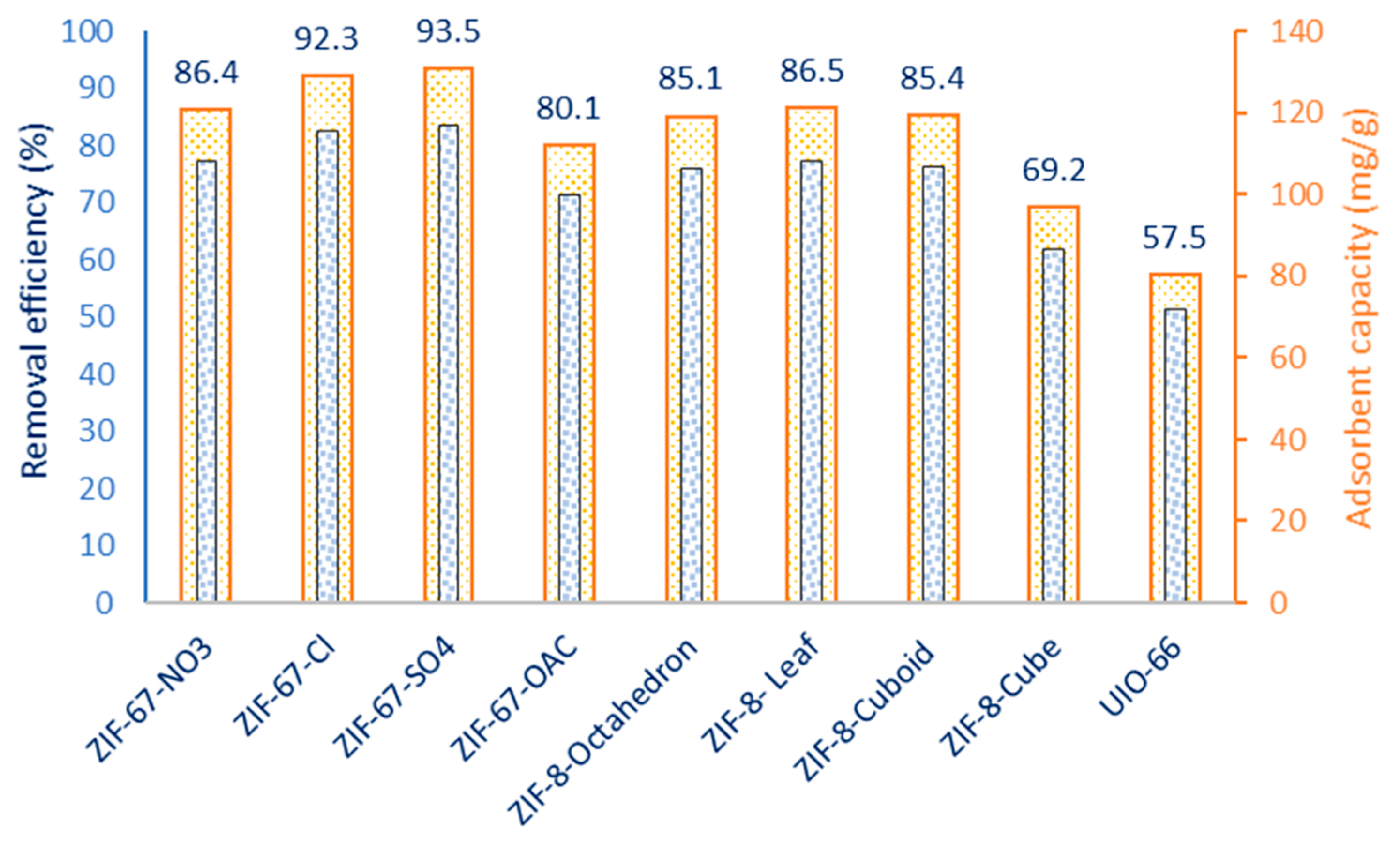
3.2. Study of MOFs for Ciprofloxacin (CIP) Removal
3.3. Model Development Using Response Surface Methodology (RSM)
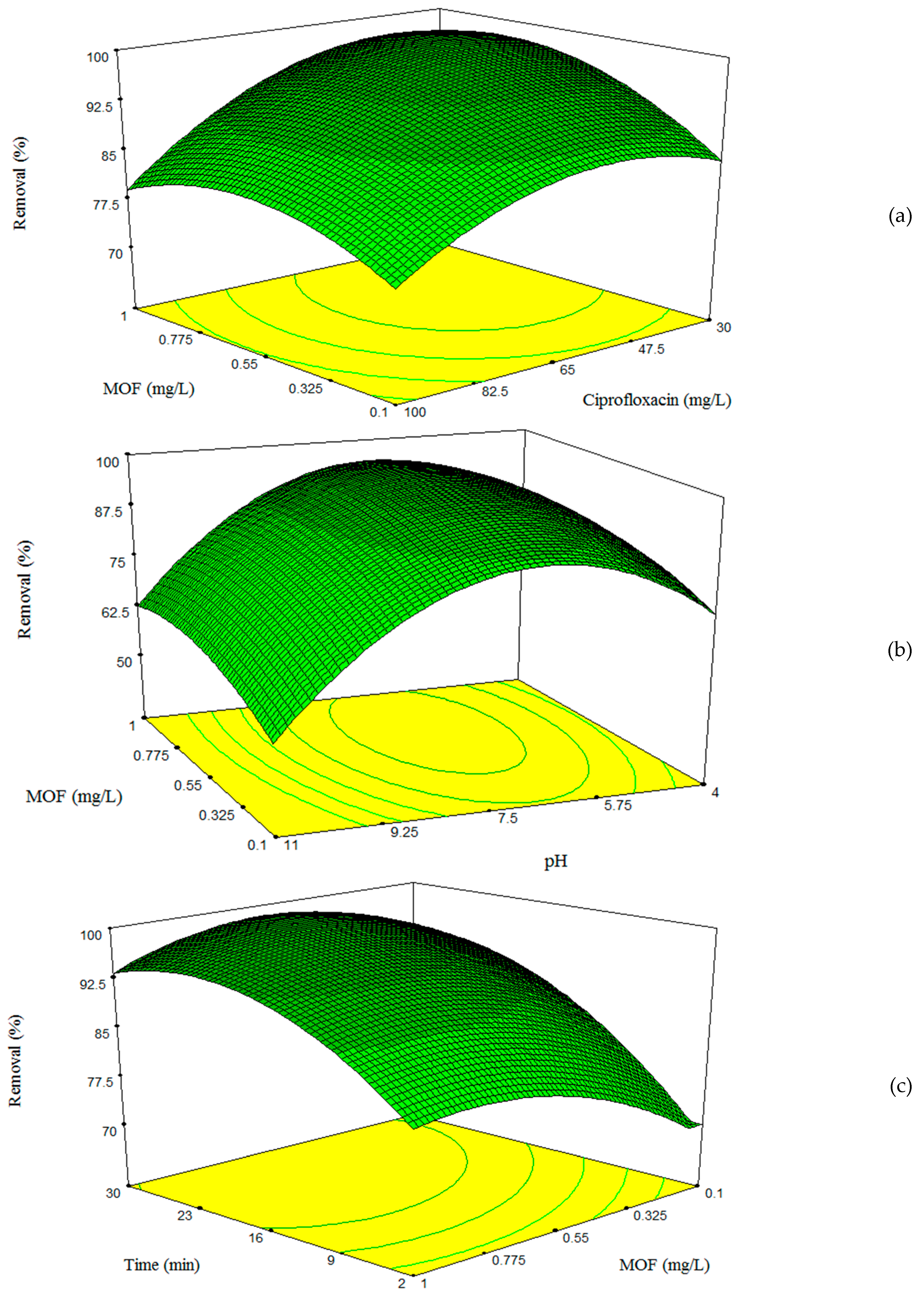
3.4. Effects of Model Variables and Their Interactions
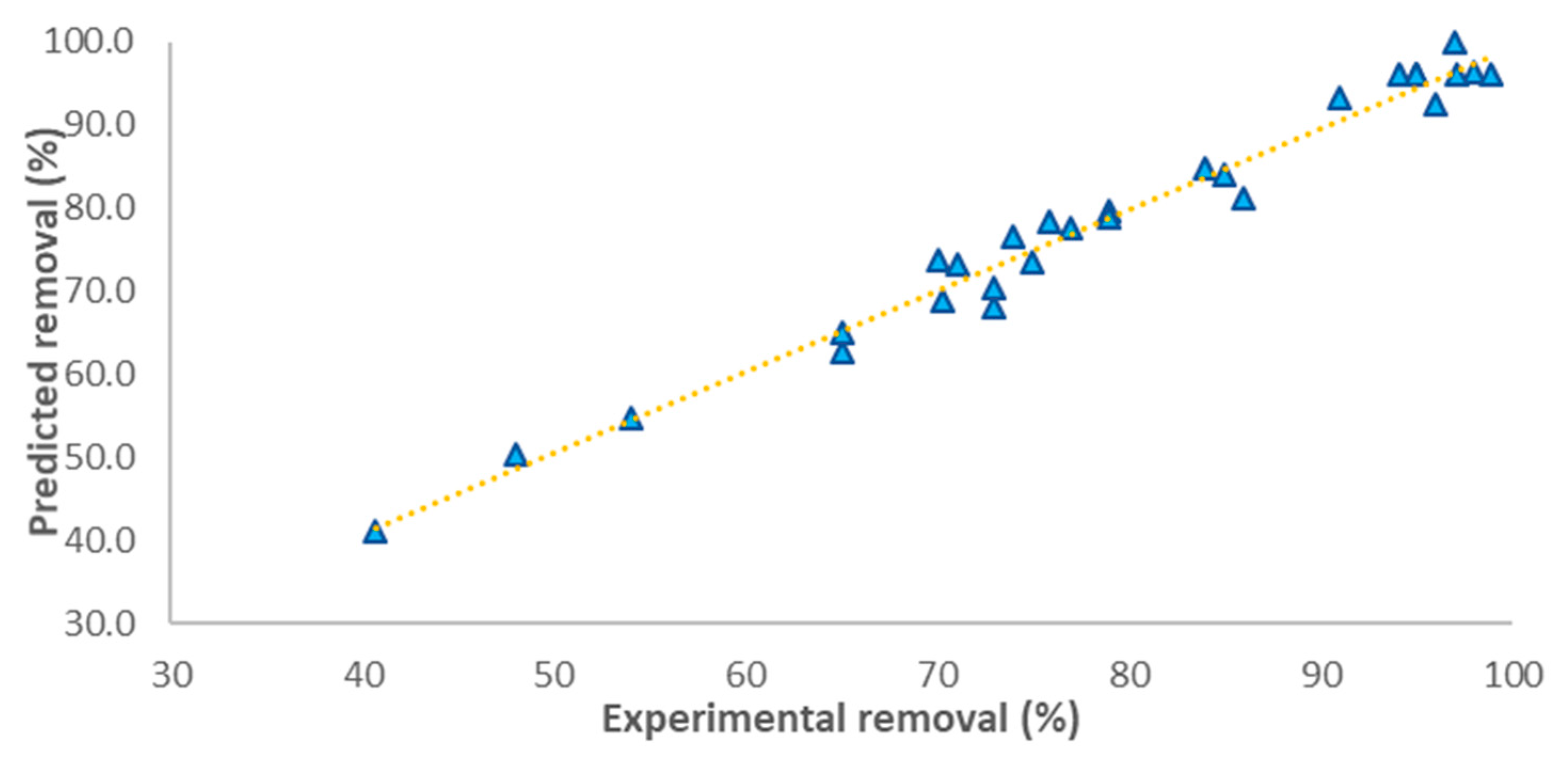
3.5. Model Optimization and Adequacy Checking
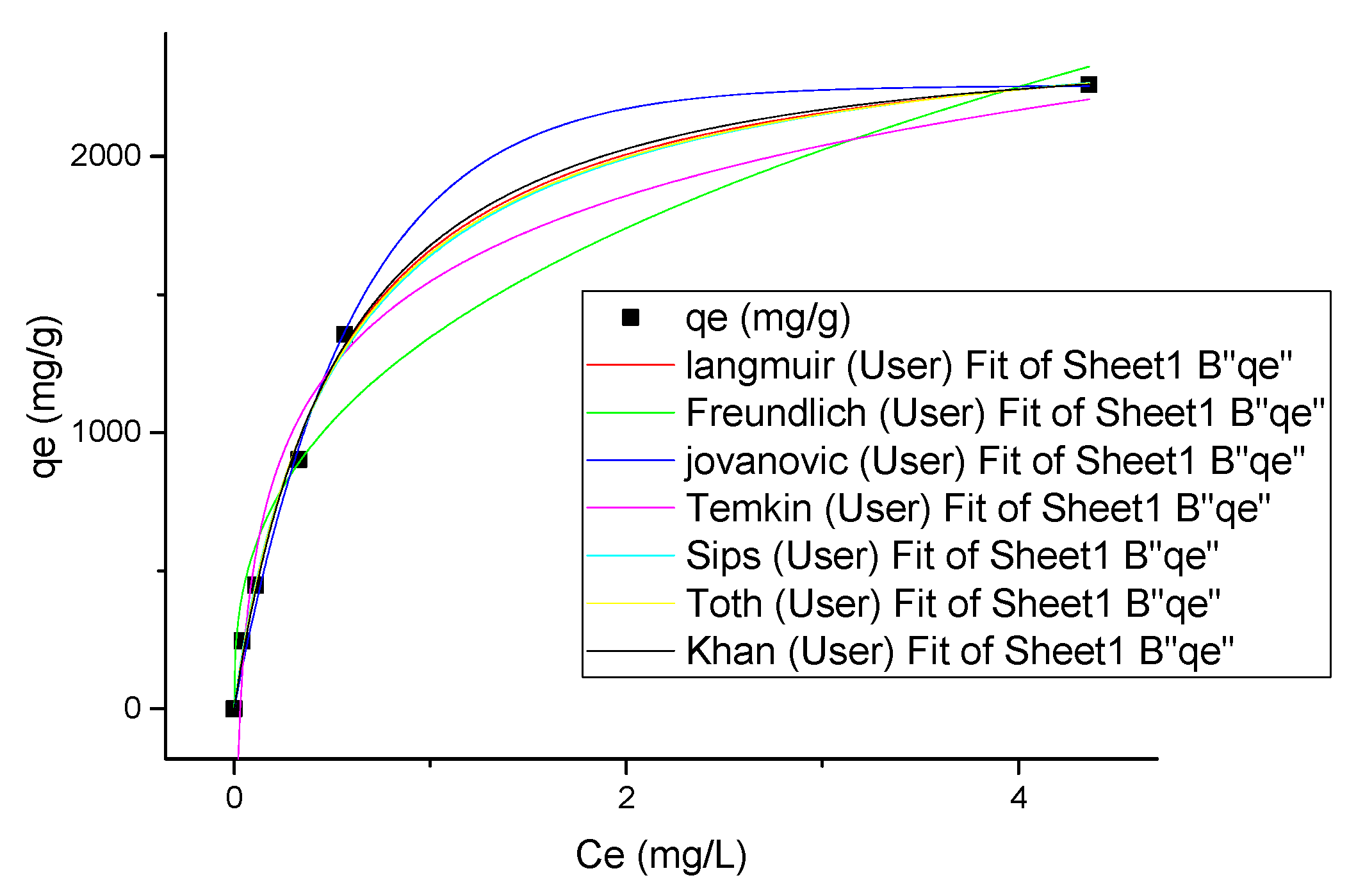
3.6. Isotherm Modeling
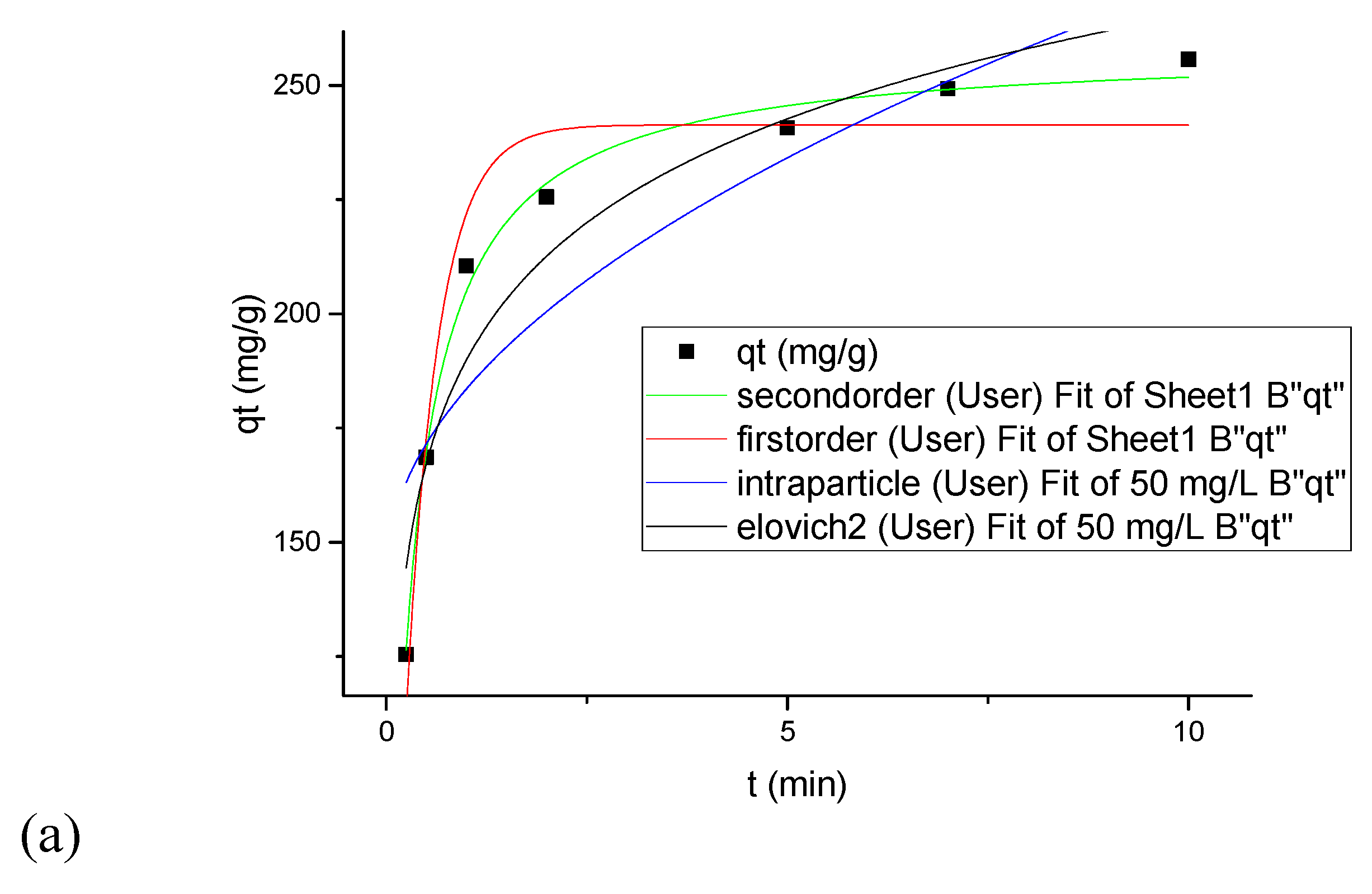
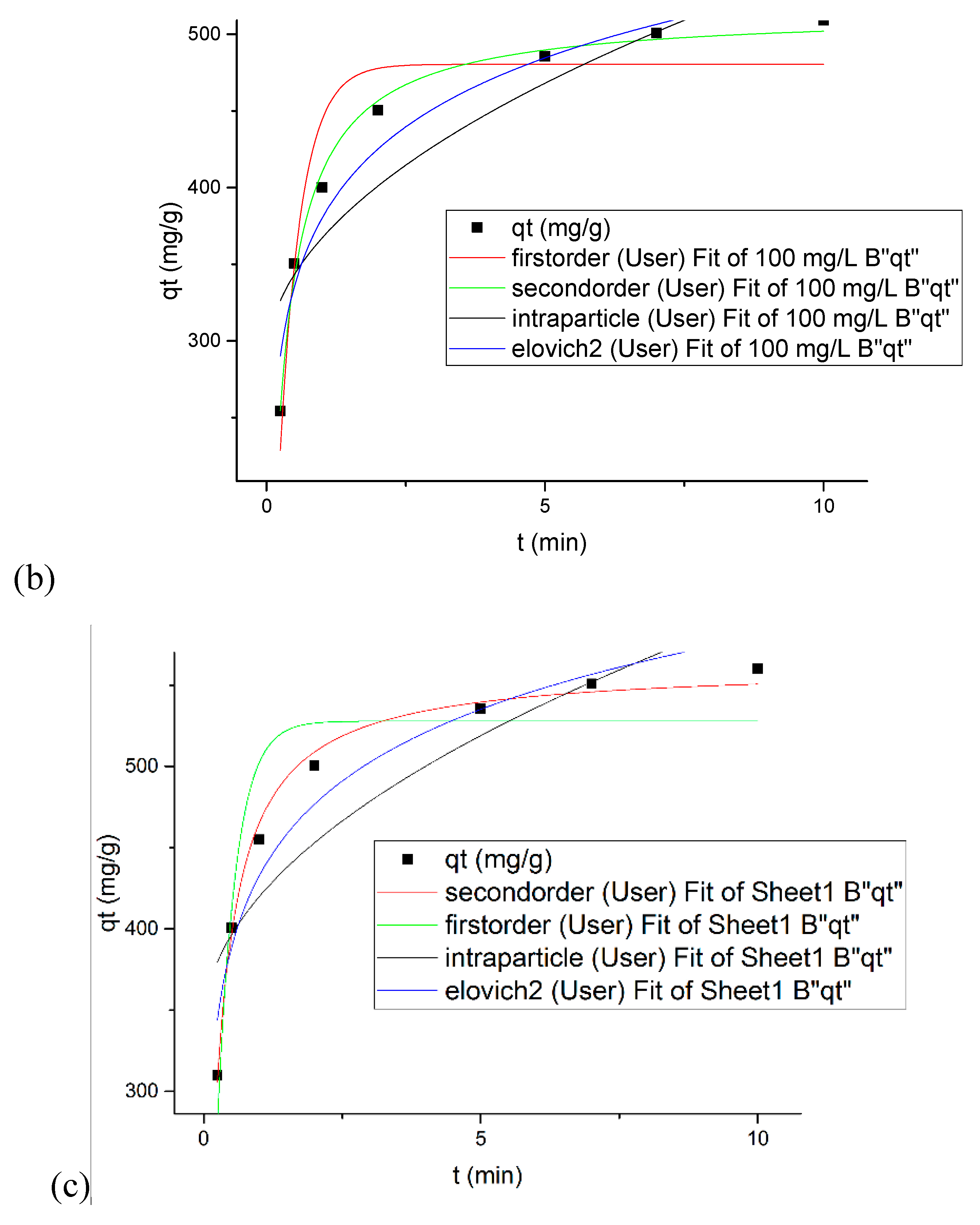
3.7. Kinetics Modeling under Convectional Mixing and Sonication
3.8. Thermodynamic of Adsorption
3.9. ZIF-67-SO4 Structural Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal–organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Bu, X.-H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Yu, F.; Sun, S.; Han, S.; Zheng, J.; Ma, J. Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 2016, 285, 588–595. [Google Scholar] [CrossRef]
- Wang, F.; Yang, B.; Wang, H.; Song, Q.; Tan, F.; Cao, Y. Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. J. Mol. Liq. 2016, 222, 188–194. [Google Scholar] [CrossRef]
- Abu Tarboush, B.J.; Chouman, A.; Jonderian, A.; Ahmad, M.; Hmadeh, M.; Al-Ghoul, M. Metal–Organic Framework-74 for Ultratrace Arsenic Removal from Water: Experimental and Density Functional Theory Studies. ACS Appl. Nano Mater. 2018, 1, 3283–3292. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Yang, C.-X.; Yan, X.-P. Metal-organic framework-801 for efficient removal of fluoride from water. Microporous Mesoporous Mater. 2018, 259, 163–170. [Google Scholar] [CrossRef]
- Ayati, A.; Shahrak, M.N.; Tanhaei, B.; Sillanpää, M. Emerging adsorptive removal of azo dye by metal–organic frameworks. Chemosphere 2016, 160, 30–44. [Google Scholar] [CrossRef]
- Ezugwu, C.I.; Asraf, M.A.; Li, X.; Liu, S.; Kao, C.-M.; Zhuiykov, S.; Verpoort, F. Selective and adsorptive removal of anionic dyes and CO2 with azolium-based metal-organic frameworks. J. Colloid Interface Sci. 2018, 519, 214–223. [Google Scholar] [CrossRef]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Liu, B.; Yang, F.; Zou, Y.; Peng, Y. Adsorption of Phenol and p-Nitrophenol from Aqueous Solutions on Metal–Organic Frameworks: Effect of Hydrogen Bonding. J. Chem. Eng. Data 2014, 59, 1476–1482. [Google Scholar] [CrossRef]
- Dehghan, A.; Zarei, A.; Jaafari, J.; Shams, M.; Mousavi Khaneghah, A. Tetracycline removal from aqueous solutions using zeolitic imidazolate frameworks with different morphologies: A mathematical modeling. Chemosphere 2019, 217, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Han, X.; Gu, P.; Fang, S.; Bai, J. Response surface methodology approach for optimization of ciprofloxacin adsorption using activated carbon derived from the residue of desilicated rice husk. J. Mol. Liq. 2017, 238, 316–325. [Google Scholar] [CrossRef]
- Guo, X.; Xing, T.; Lou, Y.; Chen, J. Controlling ZIF-67 crystals formation through various cobalt sources in aqueous solution. J. Solid State Chem. 2016, 235, 107–112. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Shahsavani, A.; Kamarehie, B.; Jafari, A.; Ghaderpoori, M.; Amini, M.M.; Ghaderpoury, A. Highly efficient adsorption of fluoride from aqueous solution by metal organic frameworks modeling, isotherms, and kinetics. Fluoride 2018, 51, 355–365. [Google Scholar]
- Liu, B.; Jian, M.; Liu, R.; Yao, J.; Zhang, X. Highly efficient removal of arsenic(III) from aqueous solution by zeolitic imidazolate frameworks with different morphology. Colloids Surf. A 2015, 481, 358–366. [Google Scholar] [CrossRef]
- Ghadiri, S.K.; Nasseri, S.; Nabizadeh, R.; Khoobi, M.; Nazmara, S.; Mahvi, A.H. Adsorption of nitrate onto anionic bio-graphene nanosheet from aqueous solutions: Isotherm and kinetic study. J. Mol. Liq. 2017, 242, 1111–1117. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Zarei, A.; Mesdaghinia, A.; Nabizadeh, R.; Alimohammadi, M.; Afsharnia, M. Response surface modeling, isotherm, thermodynamic and optimization study of arsenic (V) removal from aqueous solutions using modified bentonite-chitosan (MBC). Korean J. Chem. Eng. 2017, 34, 757–767. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Dehghan, A.; Alidadi, H.; Dolatabadi, M.; Mehrabpour, M.; Converti, A. Removal of methylene blue dye from aqueous solutions by a new chitosan/zeolite composite from shrimp waste: Kinetic and equilibrium study. Korean J. Chem. Eng. 2017, 34, 1699–1707. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. A remarkable adsorbent for removal of contaminants of emerging concern from water: Porous carbon derived from metal azolate framework-6. J. Hazard. Mater. 2017, 340, 179–188. [Google Scholar] [CrossRef]
- Goudarzi, G.; Geravandi, S.; Mohammadi, M.J.; Vosoughi, M.; Angali, K.A.; Zallaghi, E.; Neisi, A.K.; Saeidimehr, S.; Mohammadi, B. Total number of deaths and respiratory mortality attributed to particulate matter (PM 10) in Ahvaz, Iran during 2009. Int. J. Environ. Health Eng. 2015, 4, 33. [Google Scholar]
- Peng, X.; Hu, F.; Lam, F.L.Y.; Wang, Y.; Liu, Z.; Dai, H. Adsorption behavior and mechanisms of ciprofloxacin from aqueous solution by ordered mesoporous carbon and bamboo-based carbon. J. Colloid Interface Sci. 2015, 460, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Dehghan, A.; Najafpoor, A. Removing Reactive Red 120 and 196 using chitosan/zeolite composite from aqueous solutions: Kinetics, isotherms, and process optimization. J. Ind. Eng. Chem. 2017, 51, 185–195. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Giri Nandagopal, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, Y.; Tao, Y.; Fan, S.; Chu, D.-T.; Ye, X.; Ye, M.; Xie, G. Ultrasound assisted adsorption and desorption of blueberry anthocyanins using macroporous resins. Ultrason. Sonochem. 2018, 48, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A. Ultrasound wave assisted adsorption of congo red using gold-magnetic nanocomposite loaded on activated carbon: Optimization of process parameters. Ultrason. Sonochem. 2018, 46, 99–105. [Google Scholar] [CrossRef]
- Shams, M.; Dehghani, M.H.; Nabizadeh, R.; Mesdaghinia, A.; Alimohammadi, M.; Najafpoor, A.A. Adsorption of phosphorus from aqueous solution by cubic zeolitic imidazolate framework-8: Modeling, mechanical agitation versus sonication. J. Mol. Liq. 2016, 224, 151–157. [Google Scholar] [CrossRef]
- Dastkhoon, M.; Ghaedi, M.; Asfaram, A.; Goudarzi, A.; Mohammadi, S.M.; Wang, S. Improved adsorption performance of nanostructured composite by ultrasonic wave: Optimization through response surface methodology, isotherm and kinetic studies. Ultrason. Sonochem. 2017, 37, 94–105. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Zhang, L.; Zheng, S.; Zhang, Y. Enhanced boron adsorption onto synthesized MgO nanosheets by ultrasonic method. Ultrason. Sonochem 2017, 34, 938–946. [Google Scholar] [CrossRef]
- Dotto, G.L.; Santos, J.M.N.; Rodrigues, I.L.; Rosa, R.; Pavan, F.A.; Lima, E.C. Adsorption of Methylene Blue by ultrasonic surface modified chitin. J. Colloid Interface Sci. 2015, 446, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Mutavdžić Pavlović, D.; Ćurković, L.; Grčić, I.; Šimić, I.; Župan, J. Isotherm, kinetic, and thermodynamic study of ciprofloxacin sorption on sediments. Environ. Sci. Pollut. Res. Int. 2017, 24, 10091–10106. [Google Scholar] [CrossRef] [PubMed]


| Ciprofloxacin Structure | Molecular Formula | pKa |
|---|---|---|
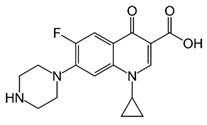 | C17H18FN3O3 | pKa1 = 5.9 |
| pKa2 = 8.9 |
| MOFs | Ligand | Metal Source | Ligand/ Metal Mole Ratio | Structural Morphology | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|---|---|
| UIO-66 | Terephthalic acid | ZrCl4 | 1 | Plate | 765 | 0.44 |
| ZIF-67 | 2-methylimidazole | Co(NO3)2 | 20 | Granular | 734 | 0.34 |
| 2-methylimidazole | Co(OAC)2 | 20 | Rhombic Dodecahedron | 1323 | 0.57 | |
| 2-methylimidazole | CoSO4 | 20 | Rhombic Dodecahedron | 1375 | 0.62 | |
| 2-methylimidazole | CoCl2 | 20 | Rhombic Dodecahedron | 1278 | 0.52 | |
| ZIF-8 | 2-methylimidazole | Zn(NO3)2 | 29.4 | Octahedron | 1151.2 | 0.58 |
| 2-methylimidazole | Zn(OAc)2 | 7.9 | Leaf | 12.7 | 0.04 | |
| 2-methylimidazole | Zn(NO3)2 | 7.9 | Cuboid | 890.4 | 0.48 | |
| 2-methylimidazole | Zn(NO3)2 | 2 | Cube | 978 | 0.51 |
| Factor | Variable Level | |||
|---|---|---|---|---|
| Code | −1 | 0 | +1 | |
| contact time (min) | X1 | 2 | 16 | 30 |
| MOF dosage (g/L) | X2 | 0.1 | 0.55 | 1 |
| pH | X3 | 4 | 7.5 | 11 |
| ciprofloxacin (mg/L) | X4 | 30 | 62.5 | 100 |
| Run No. | Coded Variable | Response (% Removal) | Run No | Coded Variable | Response (% Removal) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Observed | Predicted | X1 | X2 | X3 | X4 | Observed | Predicted | ||
| 1 | −1 | 1 | 0 | 0 | 80 | 81 | 16 | 1 | 0 | 0 | −1 | 97 | 100 |
| 2 | −1 | −1 | 0 | 0 | 73 | 68.2 | 17 | −1 | 0 | 0 | 1 | 70 | 68.9 |
| 3 | 0 | 1 | −1 | 0 | 79 | 79.7 | 18 | 0 | 0 | 0 | 0 | 94 | 96 |
| 4 | 1 | 0 | 1 | 0 | 75 | 73.6 | 19 | 0 | 1 | 0 | 1 | 79 | 79 |
| 5 | 0 | 1 | 1 | 0 | 65 | 62.7 | 20 | 1 | 0 | −1 | 0 | 77 | 77.5 |
| 6 | 0 | 0 | 1 | 1 | 48 | 50.4 | 21 | 1 | −1 | 0 | 0 | 96 | 92.5 |
| 7 | 0 | −1 | 0 | 1 | 74 | 76.6 | 22 | 0 | 0 | 0 | 0 | 95 | 96 |
| 8 | 0 | 1 | 0 | −1 | 98 | 96.2 | 23 | 0 | 0 | 1 | −1 | 65 | 65.1 |
| 9 | 0 | 0 | 0 | 0 | 95 | 96 | 24 | −1 | 0 | −1 | 0 | 71 | 73.3 |
| 10 | −1 | 0 | 1 | 0 | 41 | 41.1 | 25 | −1 | 0 | 0 | −1 | 76 | 78.4 |
| 11 | 1 | 1 | 0 | 0 | 91 | 93.4 | 26 | 0 | 0 | −1 | −1 | 86 | 81.2 |
| 12 | 0 | −1 | −1 | 0 | 70 | 73.9 | 27 | 0 | 0 | −1 | 1 | 73 | 70.4 |
| 13 | 0 | 0 | 0 | 0 | 97 | 96 | 28 | 1 | 0 | 0 | 1 | 85 | 84 |
| 14 | 0 | −1 | 1 | 0 | 54 | 54.8 | 29 | 0 | −1 | 0 | −1 | 84 | 84.9 |
| 15 | 0 | 0 | 0 | 0 | 99 | 96 | - | - | - | - | - | - | - |
| Model Term | Coefficient Estimate | Std. Error | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 96.02 | 1.47 | 65.30 | <0.0001 |
| 9.18 | 0.95 | 9.68 | 1.40 × 10−7 | |
| 3.42 | 0.95 | 3.60 | 0.0028997 | |
| −9.03 | 0.95 | −9.51 | 1.74 × 10−7 | |
| −6.38 | 0.95 | −6.72 | 9.84 × 10−6 | |
| −3.00 | 1.64 | −1.82 | 0.0894262 | |
| 7.08 | 1.64 | 4.30 | 0.0007283 | |
| −1.63 | 1.64 | −0.99 | 0.3396952 | |
| 0.50 | 1.64 | 0.30 | 0.7654894 | |
| −2.25 | 1.64 | −1.37 | 0.1926667 | |
| −1.00 | 1.64 | −0.61 | 0.5527336 | |
| −6.82 | 1.29 | −5.28 | 0.000116 | |
| −5.42 | 1.29 | −4.20 | 0.0008955 | |
| −22.83 | 1.29 | −17.69 | 5.66 × 10−11 | |
| −6.41 | 1.29 | −4.96 | 0.0002087 |
| Factor | Time (min) | MOF Dose (g/L) | pH | CIP (mg/L) | Removal (%) | |
|---|---|---|---|---|---|---|
| Predicted | Experimental | |||||
| Value | 30 | 0.22 | 7.31 | 100 | 100 | 99.9 |
| Isotherm | Parameters | Values |
|---|---|---|
| Langmuir | b (L/mg) | 1.89166 |
| qe (mg/g) | 2537.52777 | |
| χ2 | 3024.69308 | |
| SSE | 12,098.77233 | |
| 0.99567 | ||
| Freundlich | Kf (mg/g)/(mg)1/n | 1345.1812 |
| n | 2.69053 | |
| χ2 | 30,900.26595 | |
| SSE | 123,601.06379 | |
| 0.9558 | ||
| Jovanovic | qm (mg·g−1) | 2256.44168 |
| Kj (L·mg−1) | −1.64853 | |
| χ2 | 4439.2249 | |
| SSE | 17,756.89959 | |
| 0.99365 | ||
| Temkin | AT (L/mg) | 31.62846 |
| bT | 447.91592 | |
| B (J/mol) | - | |
| χ2 | 15,237.21971 | |
| SSE | 60,948.87886 | |
| 0.9782 | ||
| Sips | qms (mg/g) | 2593.86316 |
| KS (L/mg)ms | 1.71831 | |
| ms | 0.94708 | |
| χ2 | 3786.66401 | |
| SSE | 11,359.99203 | |
| 0.99458 | ||
| Toth | KT | 2577.35956 |
| AT | 0.52871 | |
| TT | 0.94943 | |
| χ2 | 3995.17545 | |
| SSE | 11,985.52634 | |
| 0.99428 | ||
| Khan | qs (mg/g) | 2772.45949 |
| bK | 1.67977 | |
| aK | 1.03572 | |
| χ2 | 3999.75536 | |
| SSE | 11,999.26609 | |
| 0.99428 |
| Concentration (mg/L) | 50 | 100 | 100 |
|---|---|---|---|
| Agitation Type | Magnetic Stirrer | Sonication | |
| qe, exp (mg/g) | 256.4 | 509.06 | 560 |
| Pseudo-First Order | |||
| qe (mg/g) | 6.13223 | 480.42467 | 527.8629 |
| k1 (min−1) | 2.51783 | 2.58553 | 3.0163 |
| χ2 | 156.19152 | 918.66385 | 1109.7541 |
| SSE | 780.95761 | 4593.31927 | 5548.7708 |
| 0.93144 | 0.89479 | 0.86801 | |
| Pseudo-Second Order | |||
| qe (mg/g) | 258.37326 | 514.80069 | 562.129 |
| k2 (g/mg·min) | 0.01483 | 0.0076 | 0.00849 |
| χ2 | 16.21654 | 62.69378 | 65.9758 |
| SSE | 81.08269 | 313.4689 | 329.8794 |
| 0.99288 | 0.99282 | 0.99215 | |
| Intraparticle Diffusion | |||
| k3 | 40.94423 | 81.66703 | 80.3091 |
| C | 142.60978 | 285.45518 | 339.36581 |
| χ2 | 618.10256 | 2061.48156 | 1950.1885 |
| SSE | 3090.51278 | 10,307.40782 | 9750.9426 |
| 0.72867 | 0.76391 | 0.7680 | |
| Elovich | |||
| a | 10,708.00341 | 22,609.6118 | 56,005.9375 |
| b | 0.03048 | 0.0154 | 0.0156 |
| χ2 | 214.18997 | 605.792 | 558.3242 |
| SSE | 1070.94984 | 3028.9600 | 2791.6209 |
| 0.90598 | 0.9306 | 0.9335 | |
| Temperature K | Ce mg/L | −∆G° kJ/mol | ∆H° KJ/mol | ∆S° KJ/mol.K |
|---|---|---|---|---|
| 293 | 3.65 | 7.88 | 58.9 | 0.23 |
| 303 | 2.55 | 9.12 | ||
| 313 | 0.7 | 12.88 | ||
| 323 | 0.5 | 14.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehghan, A.; Mohammadi, A.A.; Yousefi, M.; Najafpoor, A.A.; Shams, M.; Rezania, S. Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study. Nanomaterials 2019, 9, 1422. https://doi.org/10.3390/nano9101422
Dehghan A, Mohammadi AA, Yousefi M, Najafpoor AA, Shams M, Rezania S. Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study. Nanomaterials. 2019; 9(10):1422. https://doi.org/10.3390/nano9101422
Chicago/Turabian StyleDehghan, Aliakbar, Ali Akbar Mohammadi, Mahmood Yousefi, Ali Asghar Najafpoor, Mahmoud Shams, and Shahabaldin Rezania. 2019. "Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study" Nanomaterials 9, no. 10: 1422. https://doi.org/10.3390/nano9101422
APA StyleDehghan, A., Mohammadi, A. A., Yousefi, M., Najafpoor, A. A., Shams, M., & Rezania, S. (2019). Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study. Nanomaterials, 9(10), 1422. https://doi.org/10.3390/nano9101422






