A Review on the Promising Plasma-Assisted Preparation of Electrocatalysts
Abstract
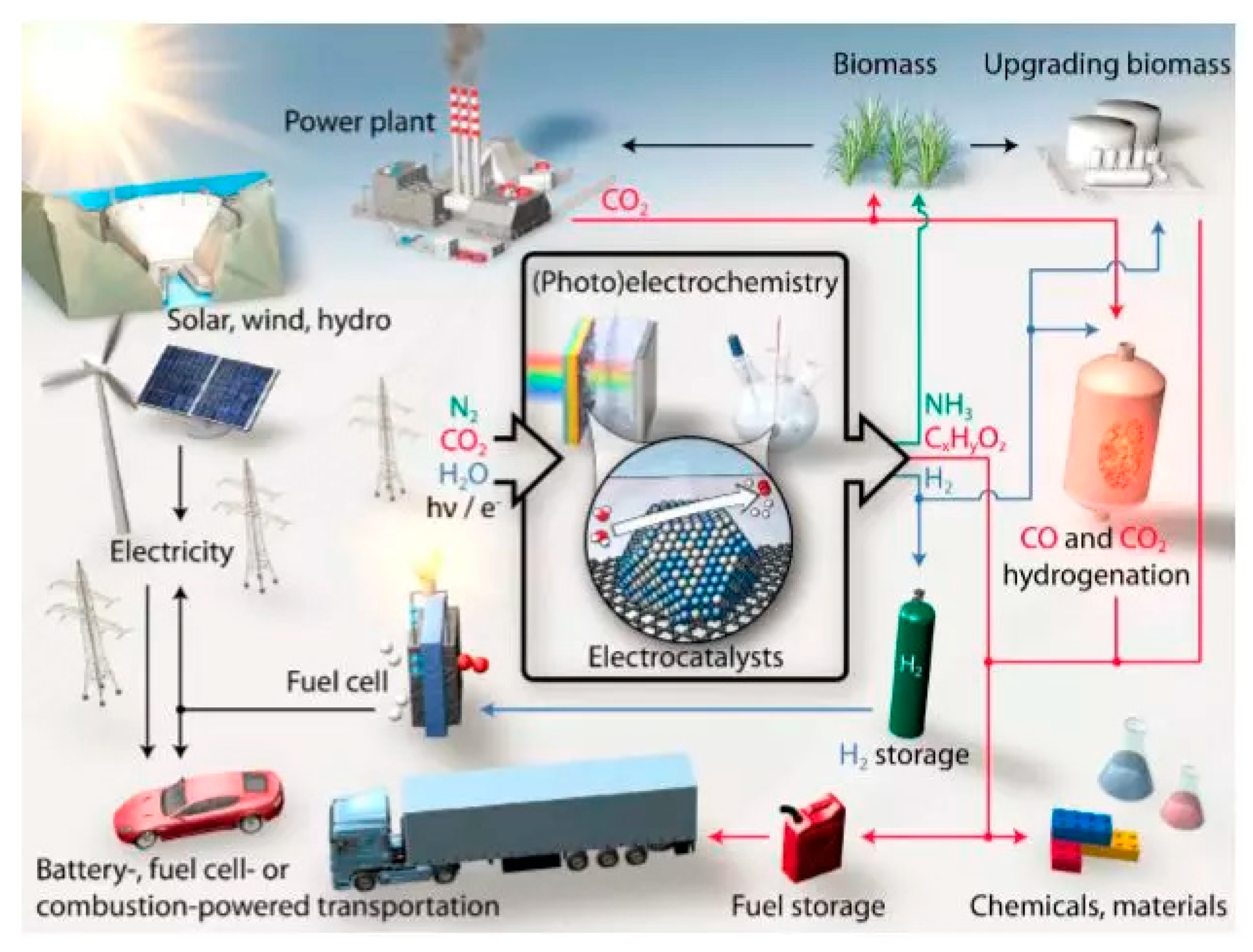
:1. Introduction
2. Noble Metal Electrocatalysts
2.1. Plasma Enhanced Deposition
2.2. Gas Plasma-Assisted Preparation
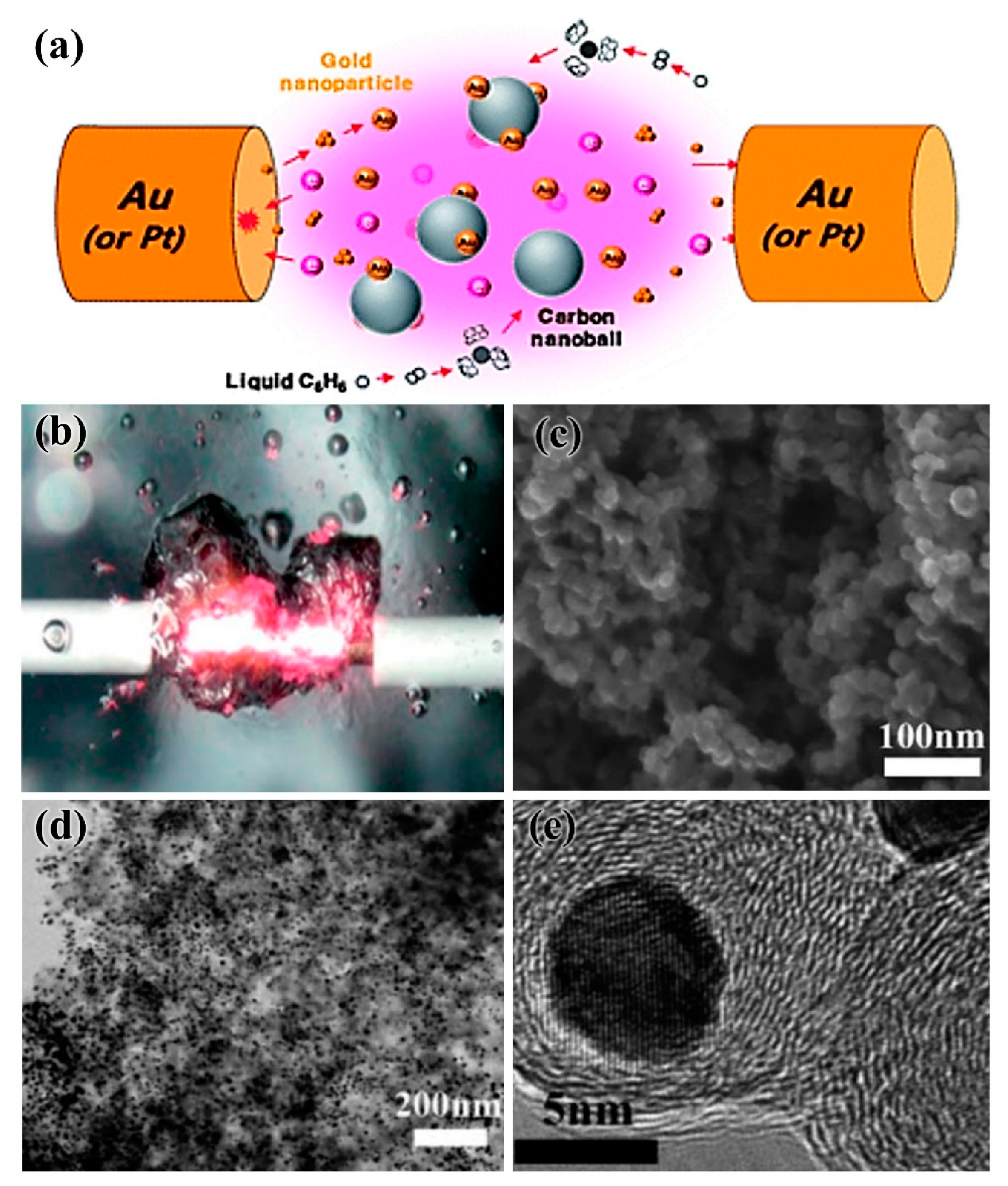
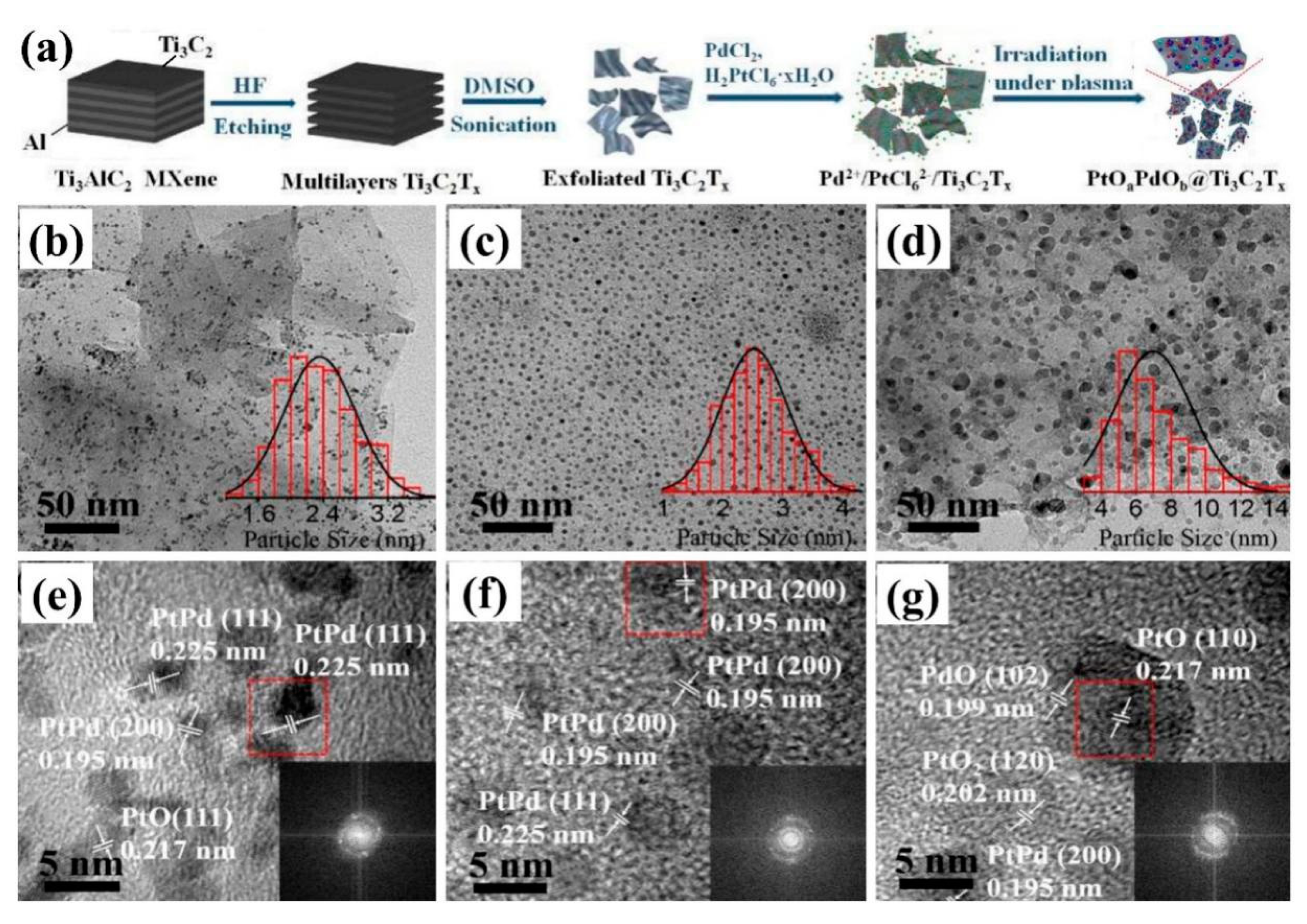
2.3. Solution Plasma Sputtering
2.4. Plasma Prepared and Modified Electrocatalysts Support
3. Non-Noble Metal Electrocatalysts
3.1. Metal and Alloy Electrocatalysts
3.2. Transition Metal Oxides
3.2.1. Cobalt Oxides
3.2.2. Perovskite-Type Oxides
3.2.3. Two-Dimensional (2D) Layered Double Hydroxides
3.2.4. Other Metal Oxides
3.3. Transition Metal Sulfides
3.4. Transition Metal Selenides
3.5. Transition Metal Nitrides
3.6. Transition Metal Phosphides
3.7. Transition Metal Carbides
3.8. Other Compounds
4. Carbon Based Electrocatalysts
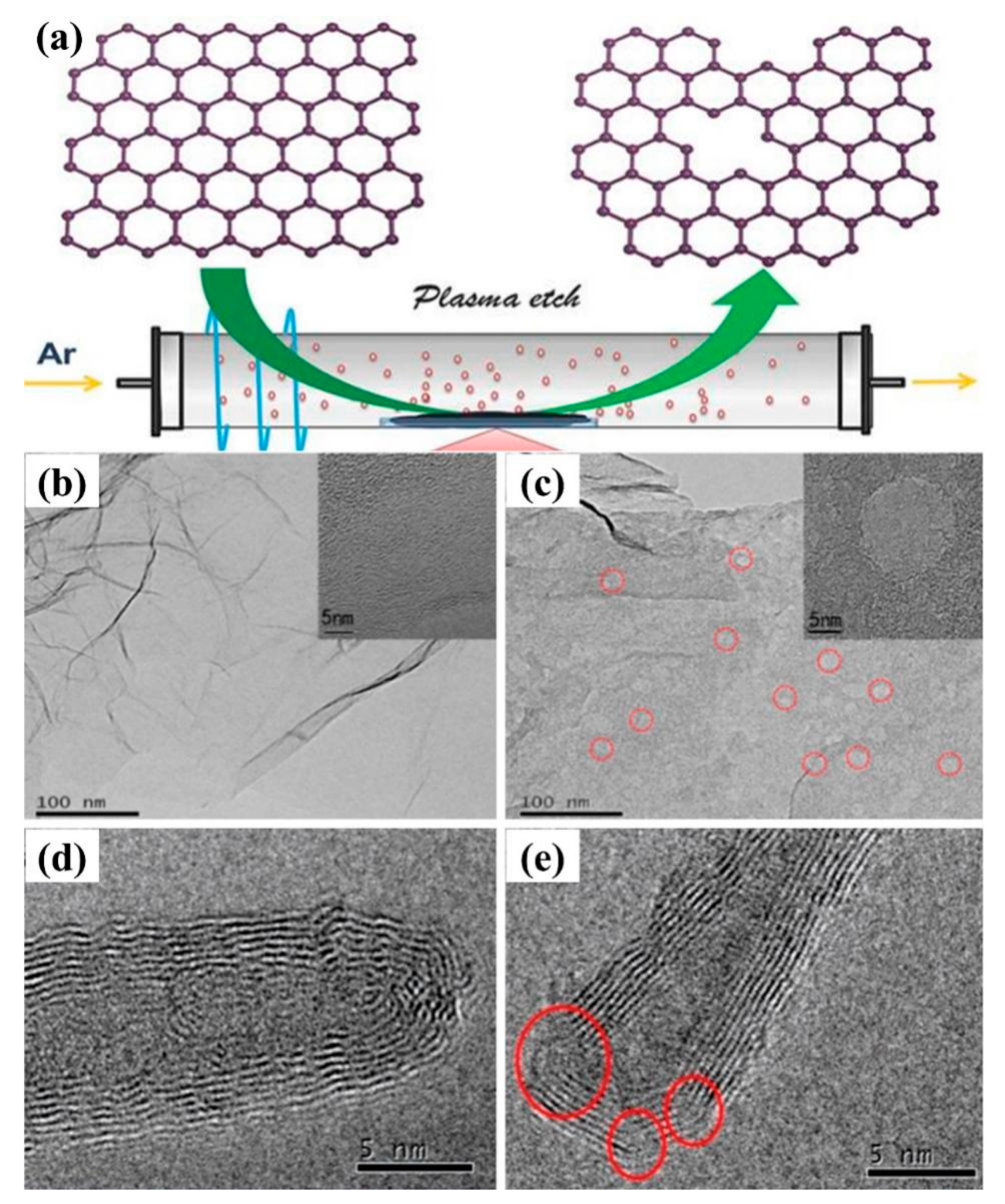
4.1. Defective Carbon Materials
4.2. Nitrogen-Doped Carbon Materials
4.3. Oxygen-Doped Carbon Materials
4.4. Sulfur-Doped Carbon Materials
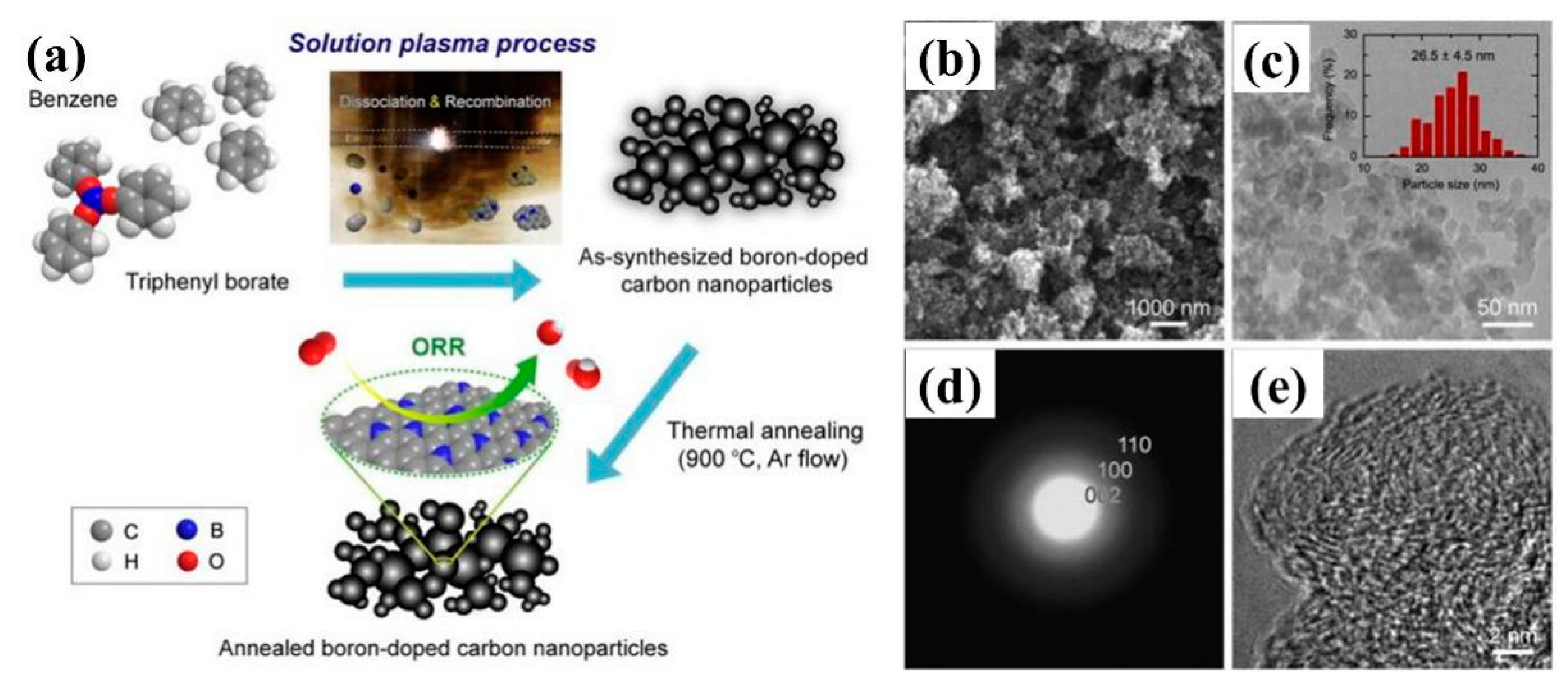
4.5. Boron-Doped Carbon Materials
4.6. Fluorine-Doped Carbon Materials
4.7. Heteroatom Co-Doped Carbon Materials
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Rao, X.; Sheng, J.; Xu, J.; Yi, J.; Liu, Y.; Zhang, J. Energy storage through CO2 electroreduction: A brief review of advanced Sn-based electrocatalysts and electrodes. J. CO2 Util. 2018, 27, 48–59. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.; Lv, X.; Zhang, L.; Zheng, G. Defect and Interface Engineering for Aqueous Electrocatalytic CO2 Reduction. Joule 2018, 2, 2251–2587. [Google Scholar] [CrossRef]
- Yan, D.F.; Li, Y.X.; Huo, J.; Chen, R.; Dai, L.M.; Wang, S.Y. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Wang, X.; Wang, S. Rational design of transition metal-based materials for highly efficient electrocatalysis. Small Methods 2018, 3, 1800211. [Google Scholar] [CrossRef]
- Vogiatzis, K.D.; Polynski, M.V.; Kirkland, J.K.; Townsend, J.; Hashemi, A.; Liu, C.; Pidko, E.A. Computational approach to molecular catalysis by 3d transition metals: Challenges and opportunities. Chem. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Tao, L.; Yan, D.; Zou, Y.; Wang, S. Recent Advances on Non-precious Metal Porous Carbon-based Electrocatalysts for Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 1775–1785. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C.-J. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Liang, H.; Ming, F.; Alshareef, H.N. Applications of Plasma in Energy Conversion and Storage Materials. Adv. Energy Mater. 2018. [Google Scholar] [CrossRef]
- Keijser, M.D.; Opdorp, C.V. Atomic layer epitaxy of gallium arsenide with the use of atomic hydrogen. Appl. Phys. Lett. 1991, 58, 1187–1189. [Google Scholar] [CrossRef]
- Heil, S.B.S.; Hemmen, J.L.V.; Hodson, C.J.; Singh, N.; Klootwijk, J.H.; Roozeboom, F.; Sanden, M.C.M.V.D.; Kessels, W.M.M. Deposition of TiN and HfO2 in a commercial 200mm remote plasma atomic layer deposition reactor. J. Vac. Sci. Technol. A 2007, 25, 1357–1366. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; Sanden, M.C.M.V.D.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A 2011, 29, 050801. [Google Scholar] [CrossRef] [Green Version]
- Ting, C.-C.; Liu, C.-H.; Tai, C.-Y.; Hsu, S.-C.; Chao, C.-S.; Pan, F.-M. The size effect of titania-supported Pt nanoparticles on the electrocatalytic activity towards methanol oxidation reaction primarily via the bifunctional mechanism. J. Power Sources 2015, 280, 166–172. [Google Scholar] [CrossRef]
- Yoshiaki, A.; Hiroyuki, T.; Shigemitsu, T.; Satoshi, E.; Akihiro, T.; Narishi, G.; Victor, M.; Ali, A.; Saad, M.A.; Yuichiro, K.; et al. Preparation of a platinum electrocatalyst by coaxial pulse arc plasma deposition. Sci. Technol. Adv. Mater. 2015, 16, 024804. [Google Scholar]
- Grigoriev, S.A.; Fedotov, A.A.; Martemianov, S.A.; Fateev, V.N. Synthesis of nanostructural electrocatalytic materials on various carbon substrates by ion plasma sputtering of platinum metals. Russ. J. Electrochem. 2014, 50, 638–646. [Google Scholar] [CrossRef]
- Falch, A.; Lates, V.; Kriek, R.J. Combinatorial Plasma Sputtering of PtxPdy Thin Film Electrocatalysts for Aqueous SO2 Electro-oxidation. Electrocatalysis 2015, 6, 322–330. [Google Scholar] [CrossRef]
- Estevez, L.; Reed, D.; Nie, Z.; Schwarz, A.M.; Nandasiri, M.I.; Kizewski, J.P.; Wang, W.; Thomsen, E.; Liu, J.; Zhang, J.-G.; et al. Tunable Oxygen Functional Groups as Electrocatalysts on Graphite Felt Surfaces for All-Vanadium Flow Batteries. ChemSusChem 2016, 9, 1455–1461. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Miao, Y.; Lin, Y.; Li, R. Plasma synthesis of Pt nanoparticles on 3D reduced graphene oxide-carbon nanotubes nanocomposites towards methanol oxidation reaction. Appl. Surf. Sci. 2018, 450, 413–421. [Google Scholar] [CrossRef]
- Xu, J.L.; Wang, S.G.; Deng, Q.R.; Liu, Y.; Zhu, J.L.; Xu, C.B.; Wang, J.H. High-performance Pt/CNTs catalysts via hydrogen plasma for methanol electrooxidation. Nano 2014, 9, 1450018. [Google Scholar] [CrossRef]
- Sui, S.; Ma, L.; Zhai, Y. TiC supported Pt–Ir electrocatalyst prepared by a plasma process for the oxygen electrode in unitized regenerative fuel cells. J. Power Sources 2011, 196, 5416–5422. [Google Scholar] [CrossRef]
- Ipshita, B.; Kumaran, V.; Venugopal, S. Fabrication of electrodes with ultralow platinum loading by RF plasma processing of self-assembled arrays of Au@Pt nanoparticles. Nanotechnology 2016, 27, 305401. [Google Scholar]
- Koh, J.H.; Jeon, H.S.; Jee, M.S.; Nursanto, E.B.; Lee, H.; Hwang, Y.J.; Min, B.K. Oxygen Plasma Induced Hierarchically Structured Gold Electrocatalyst for Selective Reduction of Carbon Dioxide to Carbon Monoxide. J. Phys. Chem. C 2015, 119, 883–889. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Sun, D.; Zhang, R.; Lin, W.-F.; Macias-Montero, M.; Patel, J.; Askari, S.; McDonald, C.; Mariotti, D.; Maguire, P. Gold nanoparticle-polymer nanocomposites synthesized by room temperature atmospheric pressure plasma and their potential for fuel cell electrocatalytic application. Sci. Rep. 2017, 7, 46682. [Google Scholar] [CrossRef] [Green Version]
- Oi Lun, L.; Hoonseung, L.; Takahiro, I. Recent progress in solution plasma-synthesized-carbon-supported catalysts for energy conversion systems. Jpn. J. Appl. Phys. 2018, 57, 0102A2. [Google Scholar]
- Kim, S.-M.; Cho, A.-R.; Lee, S.-Y. Characterization and electrocatalytic activity of Pt–M (M=Cu, Ag, and Pd) bimetallic nanoparticles synthesized by pulsed plasma discharge in water. J. Nanopart. Res. 2015, 17, 284. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, Y.-J.; Kim, J.-W.; Lee, S.-Y. Facile synthesis of Pt–Pd bimetallic nanoparticles by plasma discharge in liquid and their electrocatalytic activity toward methanol oxidation in alkaline media. Thin Solid Films 2014, 572, 260–265. [Google Scholar] [CrossRef]
- Cho, A.-R.; Kim, S.-M.; Kim, S.-C.; Kim, J.-W.; Lee, S.-Y. The Facile Synthesis of Composition-Tunable Pt-Pd Bimetallic Nanocatalysts and Their Electrocatalytic Properties in Formic Acid. J. Nanosci. Nanotechnol. 2016, 16, 11443–11447. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Yang, B.; Su, N.; Huang, H.; Cheng, J.; Yang, H.; Saito, N. Novel synthesis of PtPd nanoparticles with good electrocatalytic activity and durability. J. Alloy. Compd. 2017, 709, 588–595. [Google Scholar] [CrossRef]
- Horiguchi, G.; Chikaoka, Y.; Shiroishi, H.; Kosaka, S.; Saito, M.; Kameta, N.; Matsuda, N. Synthesis of Pt nanoparticles as catalysts of oxygen reduction with microbubble-assisted low-voltage and low-frequency solution plasma processing. J. Power Sources 2018, 382, 69–76. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile Fabrication of Platinum-Cobalt Alloy Nanoparticles with Enhanced Electrocatalytic Activity for a Methanol Oxidation Reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Hu, X.; Zhang, J.; Huang, H.; Cheng, J.; Yu, J.; Ge, C. Plasma-induced synthesis of Pt nanoparticles supported on TiO2 nanotubes for enhanced methanol electro-oxidation. Appl. Surf. Sci. 2017, 399, 403–410. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Zhang, J.; Tang, W.; Zhu, H.; Shen, X.; Saito, N. One-step facile synthesis of carbon-supported PdAu nanoparticles and their electrochemical property and stability. J. Alloy. Compd. 2015, 619, 452–457. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. A simple synthesis method for nano-metal catalyst supported on mesoporous carbon: The solution plasma process. Nanoscale 2013, 5, 6874–6882. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Saito, N. In situ solution plasma synthesis of mesoporous nanocarbon-supported bimetallic nanoparticles. RSC Adv. 2015, 5, 29131–29134. [Google Scholar] [CrossRef]
- Sung-Min, K.; Sang-Yul, L. The plasma-induced formation of silver nanocrystals in aqueous solution and their catalytic activity for oxygen reduction. Nanotechnology 2018, 29, 085602. [Google Scholar]
- Lee, Y.-J.; Kim, S.-M.; Kim, J.-W.; Lee, S.-Y. The characterization and electrocatalytic activities of carbon-supported Pt nanoparticles synthesized by the solution plasma process. Mater. Lett. 2014, 123, 184–186. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Treshchalov, A.; Rähn, M.; Kook, M.; Merisalu, M.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Oxygen Electroreduction on Pt Nanoparticles Deposited on Reduced Graphene Oxide and N-doped Reduced Graphene Oxide Prepared by Plasma-assisted Synthesis in Aqueous Solution. ChemElectroChem 2018, 5, 2902–2911. [Google Scholar] [CrossRef]
- Cui, B.; Hu, B.; Liu, J.; Wang, M.; Song, Y.; Tian, K.; Zhang, Z.; He, L. Solution-Plasma-Assisted Bimetallic Oxide Alloy Nanoparticles of Pt and Pd Embedded within Two-Dimensional Ti3C2Tx Nanosheets as Highly Active Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 23858–23873. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Ma, X.; Hu, J. Silver nanoparticles supported on graphitization micro-diamond as an electrocatalyst in alkaline medium. Vacuum 2018, 155, 380–386. [Google Scholar] [CrossRef]
- Ding, G.; Jiao, W.; Wang, R.; Niu, Y.; Chen, L.; Hao, L. Ultrafast, Reversible Transition of Superwettability of Graphene Network and Controllable Underwater Oil Adhesion for Oil Microdroplet Transportation. Adv. Funct. Mater. 2018, 28, 1706686. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Zhu, Y.; Liang, Z.; Pei, A.; Wu, C.-L.; Wang, H.; Lee, H.R.; Liu, K.; Chu, S.; et al. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat. Catal. 2018, 1, 592–600. [Google Scholar] [CrossRef]
- Chetty, R.; Maniam, K.K.; Schuhmann, W.; Muhler, M. Oxygen-Plasma-Functionalized Carbon Nanotubes as Supports for Platinum–Ruthenium Catalysts Applied in Electrochemical Methanol Oxidation. ChemPlusChem 2015, 80, 130–135. [Google Scholar] [CrossRef]
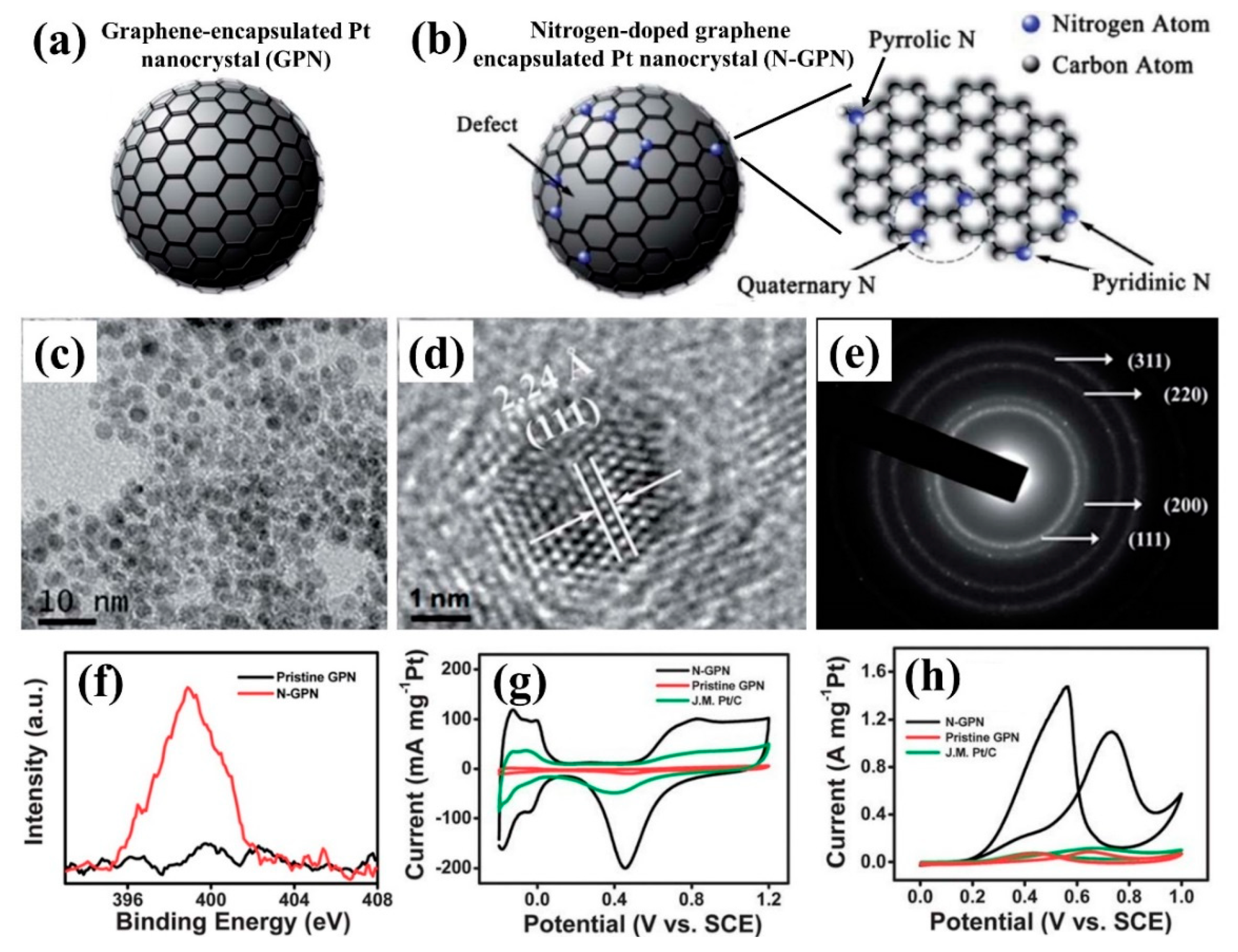
- Ding, D.; Song, Z.-L.; Cheng, Z.-Q.; Liu, W.-N.; Nie, X.-K.; Bian, X.; Chen, Z.; Tan, W. Plasma-assisted nitrogen doping of graphene-encapsulated Pt nanocrystals as efficient fuel cell catalysts. J. Mater. Chem. A 2014, 2, 472–477. [Google Scholar] [CrossRef]
- Loganathan, K.; Bose, D.; Weinkauf, D. Surface modification of carbon black by nitrogen and allylamine plasma treatment for fuel cell electrocatalyst. Int. J. Hydrogen Energy 2014, 39, 15766–15771. [Google Scholar] [CrossRef]
- Du, S.; Lin, K.; Malladi, S.K.; Lu, Y.; Sun, S.; Xu, Q.; Steinberger-Wilckens, R.; Dong, H. Plasma nitriding induced growth of Pt-nanowire arrays as high performance electrocatalysts for fuel cells. Sci. Rep. 2014, 4, 6439. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Ge, C.; Su, N.; Huang, H.; Xu, Y.; Zhang, J.; Shi, J.; Shen, X.; Saito, N. Solution plasma synthesis of Pt/ZnO/KB for photo-assisted electro-oxidation of methanol. J. Alloy. Compd. 2017, 692, 848–854. [Google Scholar] [CrossRef]
- Flis-Kabulska, I.; Sun, Y.; Zakroczymski, T.; Flis, J. Plasma carburizing for improvement of Ni-Fe cathodes for alkaline water electrolysis. Electrochim. Acta 2016, 220, 11–19. [Google Scholar] [CrossRef]
- Jin, Q.; Ren, B.; Li, D.; Cui, H.; Wang, C. Plasma-Assisted Synthesis of Self-Supporting Porous CoNPs@C Nanosheet as Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 31913–31921. [Google Scholar] [CrossRef]
- Koo, Y.; Malik, R.; Alvarez, N.; White, L.; Shanov, V.N.; Schulz, M.; Collins, B.; Sankar, J.; Yun, Y. Aligned carbon nanotube/copper sheets: A new electrocatalyst for CO2 reduction to hydrocarbons. RSC Adv. 2014, 4, 16362–16367. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.-W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef]
- Gao, D.; Zegkinoglou, I.; Divins, N.J.; Scholten, F.; Sinev, I.; Grosse, P.; Roldan Cuenya, B. Plasma-Activated Copper Nanocube Catalysts for Efficient Carbon Dioxide Electroreduction to Hydrocarbons and Alcohols. ACS Nano 2017, 11, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.-C.; Pan, L.; Shen, G.-Q.; Mahmood, N.; Ma, Y.-H.; Shi, Y.; Jia, W.; Wang, L.; Zhang, X.; et al. Engineering Cobalt Defects in Cobalt Oxide for Highly Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2018, 8, 3803–3811. [Google Scholar] [CrossRef]
- Jozwiak, L.; Balcerzak, J.; Kubiczek, A.; Tyczkowski, J. Plasma deposited thin-film sandwich-like bifunctional electrocatalyst for oxygen reduction and evolution reactions. Thin Solid Films 2018, 660, 161–165. [Google Scholar] [CrossRef]
- Kim, S.-M.; Jo, Y.-G.; Lee, M.-H.; Saito, N.; Kim, J.-W.; Lee, S.-Y. The plasma-assisted formation of Ag@Co3O4 core-shell hybrid nanocrystals for oxygen reduction reaction. Electrochim. Acta 2017, 233, 123–133. [Google Scholar] [CrossRef]
- Taiwo, O.; Jun, M.; Yi, G.; Wei, Z.; Jian, J.; Zhao, X.S.; Zhonghua, Z. A new approach to nanoporous graphene sheets via rapid microwave-induced plasma for energy applications. Nanotechnology 2014, 25, 495604. [Google Scholar]
- Lang, X.; Zhang, Y.; Cai, K.; Li, L.; Wang, Q.; Zhang, Q. High performance CoO nanospheres catalyst synthesized by DC arc discharge plasma method as air electrode for lithium-oxygen battery. Ionics 2018, 25, 35–40. [Google Scholar] [CrossRef]
- Gan, Q.; He, H.; Zhao, K.; He, Z.; Liu, S.; Yang, S. Plasma-Induced Oxygen Vacancies in Urchin-Like Anatase Titania Coated by Carbon for Excellent Sodium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2018, 10, 7031–7042. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Zhang, B.; Wan, H.; Li, Z.; Ji, X.; Xu, K.; Chen, C.; Zha, D.; Miao, L.; et al. Synergistic effect of two actions sites on cobalt oxides towards electrochemical water-oxidation. Nano Energy 2017, 42, 98–105. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Pei, Z.; Li, H.; Wang, Z.; Liu, Z.; Tang, Z.; Zapien, J.A.; Zhi, C. Flexible Waterproof Rechargeable Hybrid Zinc Batteries Initiated by Multifunctional Oxygen Vacancies-Rich Cobalt Oxide. ACS Nano 2018, 12, 8597–8605. [Google Scholar] [CrossRef] [PubMed]
- Min, W. Plasma-induced nanoporous metal oxides with nitrogen doping for high-performance electrocatalysis. Nanotechnology 2017, 28, 242501. [Google Scholar]
- Lei, X.; Zhimin, W.; Jialu, W.; Zhaohui, X.; Xiaobing, H.; Zhigang, L.; Shuangyin, W. N-doped nanoporous Co3O4 nanosheets with oxygen vacancies as oxygen evolving electrocatalysts. Nanotechnology 2017, 28, 165402. [Google Scholar]
- Uhlig, L.M.; Sievers, G.; Brüser, V.; Dyck, A.; Wittstock, G. Characterization of different plasma-treated cobalt oxide catalysts for oxygen reduction reaction in alkaline media. Sci. Bull. 2016, 61, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Xia, C.; Emwas, A.-H.; Anjum, D.H.; Miao, X.; Alshareef, H.N. Phosphine plasma activation of α-Fe2O3 for high energy asymmetric supercapacitors. Nano Energy 2018, 49, 155–162. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Wang, Y.; Huang, Y.C.; Wei, Z.X.; Dong, C.L.; Ma, J.M.; Shen, S.H.; Li, Y.F.; Wang, S.Y. Filling the oxygen vacancies in Co3O4 with phosphorus: An ultra-efficient electrocatalyst for overall water splitting. Energy Environ. Sci. 2017, 10, 2563–2569. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Zhang, L.; Sun, X. Phosphorus-Doped Co3O4 Nanowire Array: A Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Dou, S.; Dong, C.-L.; Hu, Z.; Huang, Y.-C.; Chen, J.-l.; Tao, L.; Yan, D.; Chen, D.; Shen, S.; Chou, S.; et al. Atomic-Scale CoOx Species in Metal–Organic Frameworks for Oxygen Evolution Reaction. Adv. Funct. Mater. 2017, 27, 1702546. [Google Scholar] [CrossRef]
- Yin, W.-J.; Weng, B.; Ge, J.; Sun, Q.; Li, Z.; Yan, Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2018, 12, 442–462. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, M.; Lu, Y.-R.; Hao, L.; Liu, D.; Dong, C.-L.; Li, Y.; Wang, S. Preferential Cation Vacancies in Perovskite Hydroxide for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2018, 57, 8691–8696. [Google Scholar] [CrossRef] [PubMed]
- Hayden, B.E.; Rogers, F.K. Oxygen reduction and oxygen evolution on SrTi1−xFexO3−y (STFO) perovskite electrocatalysts. J. Electroanal. Chem. 2018, 819, 275–282. [Google Scholar] [CrossRef]
- Chen, G.; Hu, Z.; Zhu, Y.; Gu, B.; Zhong, Y.; Lin, H.-J.; Chen, C.-T.; Zhou, W.; Shao, Z. A Universal Strategy to Design Superior Water-Splitting Electrocatalysts Based on Fast In Situ Reconstruction of Amorphous Nanofilm Precursors. Adv. Mater. 2018, 30, 1804333. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.Y.; Liu, D.D.; Zou, Y.Q.; Wang, S.Y. Water-Plasma-Enabled Exfoliation of Ultrathin Layered Double Hydroxide Nanosheets with Multivacancies for Water Oxidation. Adv. Mater. 2017, 29, 1701546. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.A.; Romero, J.; Varela, M.; Hauke, F.; Abellán, G.; Hirsch, A.; Coronado, E. Alkoxide-intercalated NiFe-layered double hydroxides magnetic nanosheets as efficient water oxidation electrocatalysts. Inorg. Chem. Front. 2016, 3, 478–487. [Google Scholar] [CrossRef] [Green Version]
- Browne, M.P.; Sofer, Z.; Pumera, M. Layered and two dimensional metal oxides for electrochemical energy conversion. Energy Environ. Sci. 2018, 12, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Zhang, Y.Q.; Liu, Z.J.; Xie, C.; Feng, S.; Liu, D.D.; Shao, M.F.; Wang, S.Y. Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Xie, C.; Zhang, Z.Y.; Liu, D.D.; Chen, R.; Wang, S.Y. In Situ Exfoliated, N-Doped, and Edge-Rich Ultrathin Layered Double Hydroxides Nanosheets for Oxygen Evolution Reaction. Adv. Funct. Mater. 2018, 28, 1703363. [Google Scholar] [CrossRef]
- Oturan, N.; Ganiyu, S.O.; Raffy, S.; Oturan, M.A. Sub-stoichiometric titanium oxide as a new anode material for electro-Fenton process: Application to electrocatalytic destruction of antibiotic amoxicillin. Appl. Catal. B Environ. 2017, 217, 214–223. [Google Scholar] [CrossRef]
- Luo, X.; Lu, J.; Sohm, E.; Ma, L.; Wu, T.; Wen, J.; Qiu, D.; Xu, Y.; Ren, Y.; Miller, D.J.; et al. Uniformly dispersed FeOx atomic clusters by pulsed arc plasma deposition: An efficient electrocatalyst for improving the performance of Li–O2 battery. Nano Res. 2016, 9, 1913–1920. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Z.; Wang, Z.; Pan, Y. Multivalent manganese oxides with high electrocatalytic activity for oxygen reduction reaction. Front. Chem. Sci. Eng. 2018, 12, 790–797. [Google Scholar] [CrossRef]
- Jiang, M.; Fu, C.; Yang, J.; Liu, Q.; Zhang, J.; Sun, B. Defect-engineered MnO2 enhancing oxygen reduction reaction for high performance Al-air batteries. Energy Storage Mater. 2018, 18, 34–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, K.; Xia, X.; Zhang, Z.; Rawat, R.S.; Fan, H.J. Prereduction of Metal Oxides via Carbon Plasma Treatment for Efficient and Stable Electrocatalytic Hydrogen Evolution. Small 2018, 14, 1800340. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Kong, X.; Chen, W.; Su, H.; Liu, Y.; Cai, F.; Wang, G.; Zeng, J. Oxygen Vacancies in ZnO Nanosheets Enhance CO2 Electrochemical Reduction to CO. Angew. Chem. Int. Ed. 2018, 57, 6054–6059. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.; Liu, P.; Guo, C.; Luo, M.; Han, J.; Lin, T.; Huang, F.; Chen, M. Atomic-Sized Pores Enhanced Electrocatalysis of TaS2 Nanosheets for Hydrogen Evolution. Adv. Mater. 2016, 28, 8945–8949. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Duan, X.; Wang, C.; Duan, X.; Wang, S. Plasma-engineered MoS2 thin-film as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2015, 51, 7470–7473. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, L.; Zhang, Y.; Hu, J.; Leung, M.K.H. Janus effect of O2 plasma modification on the electrocatalytic hydrogen evolution reaction of MoS2. J. Catal. 2018, 361, 384–392. [Google Scholar] [CrossRef]
- Huang, J.; Deng, X.; Wan, H.; Chen, F.; Lin, Y.; Xu, X.; Ma, R.; Sasaki, T. Liquid Phase Exfoliation of MoS2 Assisted by Formamide Solvothermal Treatment and Enhanced Electrocatalytic Activity Based on (H3Mo12O40P/MoS2)n Multilayer Structure. ACS Sustain. Chem. Eng. 2018, 6, 5227–5237. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Lu, A.-Y.; Tseng, C.-C.; Yang, X.; Hedhili, M.N.; Chen, M.-C.; Wei, K.-H.; Li, L.-J. Activating basal-plane catalytic activity of two-dimensional MoS2 monolayer with remote hydrogen plasma. Nano Energy 2016, 30, 846–852. [Google Scholar] [CrossRef]
- Lu, A.-Y.; Yang, X.; Tseng, C.-C.; Min, S.; Lin, S.-H.; Hsu, C.-L.; Li, H.; Idriss, H.; Kuo, J.-L.; Huang, K.-W.; et al. High-Sulfur-Vacancy Amorphous Molybdenum Sulfide as a High Current Electrocatalyst in Hydrogen Evolution. Small 2016, 12, 5530–5537. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Huo, J.; Wang, S.; Dai, L. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 1320–1326. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Y.; Yu, Y.; Du, Y.; Zhang, B. Engineering Sulfur Defects, Atomic Thickness, and Porous Structures into Cobalt Sulfide Nanosheets for Efficient Electrocatalytic Alkaline Hydrogen Evolution. ACS Catal. 2018, 8, 8077–8083. [Google Scholar] [CrossRef]
- He, L.; Zhou, D.; Lin, Y.; Ge, R.; Hou, X.; Sun, X.; Zheng, C. Ultrarapid in Situ Synthesis of Cu2S Nanosheet Arrays on Copper Foam with Room-Temperature-Active Iodine Plasma for Efficient and Cost-Effective Oxygen Evolution. ACS Catal. 2018, 8, 3859–3864. [Google Scholar] [CrossRef]
- Yeo, S.; Nandi, D.K.; Rahul, R.; Kim, T.H.; Shong, B.; Jang, Y.; Bae, J.-S.; Han, J.W.; Kim, S.-H.; Kim, H. Low-temperature direct synthesis of high quality WS2 thin films by plasma-enhanced atomic layer deposition for energy related applications. Appl. Surf. Sci. 2018, 459, 596–605. [Google Scholar] [CrossRef]
- Escalera-López, D.; Griffin, R.; Isaacs, M.; Wilson, K.; Palmer, R.E.; Rees, N.V. MoS2 and WS2 nanocone arrays: Impact of surface topography on the hydrogen evolution electrocatalytic activity and mass transport. Appl. Mater. Today 2018, 11, 70–81. [Google Scholar] [CrossRef]
- Qu, S.; Chen, W.; Yu, J.; Chen, G.; Zhang, R.; Chu, S.; Huang, J.; Wang, X.; Li, C.; Ostrikov, K. Cross-linked trimetallic nanopetals for electrocatalytic water splitting. J. Power Sources 2018, 390, 224–233. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, K.; Wei, Z.; Li, H.; Zhang, T.; Li, X.; Cai, W.; Ma, J.; Fan, H.J.; Li, Y. Strong Electronic Interaction in Dual-Cation-Incorporated NiSe2 Nanosheets with Lattice Distortion for Highly Efficient Overall Water Splitting. Adv. Mater. 2018, 30, 1802121. [Google Scholar] [CrossRef]
- Deng, S.; Zhong, Y.; Zeng, Y.; Wang, Y.; Yao, Z.; Yang, F.; Lin, S.; Wang, X.; Lu, X.; Xia, X.; et al. Directional Construction of Vertical Nitrogen-Doped 1T-2H MoSe2/Graphene Shell/Core Nanoflake Arrays for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2017, 29, 1700748. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Yuan, Q.; Tang, H.; Yu, F.; Lv, X. A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water. RSC Adv. 2016, 6, 45041–45048. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Chen, S.; Rawat, R.S.; Fan, H.J. 3D Porous Hierarchical Nickel–Molybdenum Nitrides Synthesized by RF Plasma as Highly Active and Stable Hydrogen-Evolution-Reaction Electrocatalysts. Adv. Energy Mater. 2016, 6, 1600221. [Google Scholar] [CrossRef]
- Ouyang, B.; Zhang, Y.; Zhang, Z.; Fan, H.J.; Rawat, R.S. Nitrogen-Plasma-Activated Hierarchical Nickel Nitride Nanocorals for Energy Applications. Small 2017, 13, 1604265. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; He, B.; Peng, H.-Q.; Zhao, Y.; Cheng, J.; Xia, J.; Shen, J.; Ng, T.-W.; Meng, X.; Lee, C.-S.; et al. Unconventional Nickel Nitride Enriched with Nitrogen Vacancies as a High-Efficiency Electrocatalyst for Hydrogen Evolution. Adv. Sci. 2018, 5, 1800406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-C.; Liu, B.-H.; Su, C.-Y.; Liu, W.-S.; Kei, C.-C.; Wang, K.-W.; Perng, T.-P. Electronic Band Structure and Electrocatalytic Performance of Cu3N Nanocrystals. ACS Appl. Nano Mater. 2018, 1, 3673–3681. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe–N-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, J.; Zhang, P.; Deng, L.; Yao, L.; Ren, X.; Li, Y.; Mi, H.; Sun, L. Air plasma etching towards rich active sites in Fe/N-porous carbon for the oxygen reduction reaction with superior catalytic performance. J. Mater. Chem. A 2017, 5, 16605–16610. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, B.; Gao, Q.; Zheng, Y.-R.; Zheng, X.-S.; Zhu, J.-F.; Gao, M.-R.; Yu, S.-H. A Janus Nickel Cobalt Phosphide Catalyst for High-Efficiency Neutral-pH Water Splitting. Angew. Chem. Int. Ed. 2018, 57, 15445–15449. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Lin, Y.; Cao, X.; Cheng, Y.; Liu, S.; Zeng, L.; Cheong, W.-C.; Zhao, D.; Wu, K.; et al. Electronic structure and d-band center control engineering over M-doped CoP (M=Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production. Nano Energy 2019, 56, 411–419. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlögl, U.; Alshareef, H.N. Plasma-Assisted Synthesis of NiCoP for Efficient Overall Water Splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, T.; Liang, J.; Wang, N.; Kong, X.; Wang, J.; Qian, H.; Zhou, Y.; Liu, F.; Wei, C.; et al. Highly wettable and metallic NiFe-phosphate/phosphide catalyst synthesized by plasma for highly efficient oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 7509–7516. [Google Scholar] [CrossRef]
- Dinh, K.N.; Sun, X.; Dai, Z.; Zheng, Y.; Zheng, P.; Yang, J.; Xu, J.; Wang, Z.; Yan, Q. O2 plasma and cation tuned nickel phosphide nanosheets for highly efficient overall water splitting. Nano Energy 2018, 54, 82–90. [Google Scholar] [CrossRef]
- Peng, X.; Qasim, A.M.; Jin, W.; Wang, L.; Hu, L.; Miao, Y.; Li, W.; Li, Y.; Liu, Z.; Huo, K.; et al. Ni-doped amorphous iron phosphide nanoparticles on TiN nanowire arrays: An advanced alkaline hydrogen evolution electrocatalyst. Nano Energy 2018, 53, 66–73. [Google Scholar] [CrossRef]
- Goryachev, A.; Gao, L.; Zhang, Y.; Rohling, R.Y.; Vervuurt, R.H.J.; Bol, A.A.; Hofmann, J.P.; Hensen, E.J.M. Stability of CoPx Electrocatalysts in Continuous and Interrupted Acidic Electrolysis of Water. ChemElectroChem 2018, 5, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Mao, Z.; Zhao, Z.; Peng, X.; Luo, J.; Sun, X. Plasma-activated Co3(PO4)2 nanosheet arrays with Co3+-Rich surfaces for overall water splitting. J. Power Sources 2018, 400, 190–197. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, Z.; Shi, J.; Xiong, W.; Zhang, H.; Chen, Q.; Liu, Z.; Wang, X. Atomic Layer Deposition of Nickel Carbide from a Nickel Amidinate Precursor and Hydrogen Plasma. ACS Appl. Mater. Interfaces 2018, 10, 8384–8390. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Guo, Q.; Guo, Z.; Li, H.; Zhao, R.; Chen, Q.; Liu, Z.; Wang, X. Atomic layer deposition of nickel carbide for supercapacitors and electrocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 4297–4304. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Cho, J.-M.; Kim, I.; Jeong, D.S.; Lee, K.-S.; Park, J.-K.; Baik, Y.-J.; Choi, H.-J.; Lee, W.-S. Tungsten carbide nanowalls as electrocatalyst for hydrogen evolution reaction: New approach to durability issue. Appl. Catal. B Environ. 2017, 203, 684–691. [Google Scholar] [CrossRef]
- Guo, Y.; Teng, W.; Chen, J.; Jie, Z.; Ostrikov, K.K. Air Plasma Activation of Catalytic Sites in a Metal-Cyanide Framework for Efficient Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800085. [Google Scholar] [CrossRef]
- Yan, D.; Dong, C.-L.; Huang, Y.-C.; Zou, Y.; Xie, C.; Wang, Y.; Zhang, Y.; Liu, D.; Shen, S.; Wang, S. Engineering the coordination geometry of metal–organic complex electrocatalysts for highly enhanced oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 805–810. [Google Scholar] [CrossRef]
- Ghausi, M.A.; Xie, J.; Li, Q.; Wang, X.; Yang, R.; Wu, M.; Wang, Y.; Dai, L. CO2 Overall Splitting by a Bifunctional Metal-Free Electrocatalyst. Angew. Chem. Int. Ed. 2018, 57, 13135–13139. [Google Scholar] [CrossRef]
- Lai, J.; Nsabimana, A.; Luque, R.; Xu, G. 3D Porous Carbonaceous Electrodes for Electrocatalytic Applications. Joule 2018, 2, 76–93. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yu, F.; Ma, C.; Xue, X.; Fu, H.; Yuan, H.; Yang, S.; Wang, G.; Guo, X.; Zhang, L. Effective Oxygen Reduction Reaction Performance of FeCo Alloys In Situ Anchored on Nitrogen-Doped Carbon by the Microwave-Assistant Carbon Bath Method and Subsequent Plasma Etching. Nanomaterials 2019, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Jin, C.; Yuan, J.; Zhang, Z. Atomic Defects in Two-Dimensional Materials: From Single-Atom Spectroscopy to Functionalities in Opto-/Electronics, Nanomagnetism, and Catalysis. Adv. Mater. 2017, 29, 1606434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.L.; Huo, J.; Wang, S.Y.; Dai, L.M. Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Zhao, Z.H.; Wang, Y.Y.; Dou, S.; Yan, D.F.; Liu, D.D.; Xia, Z.H.; Wang, S.Y. In Situ Exfoliated, Edge-Rich, Oxygen-Functionalized Graphene from Carbon Fibers for Oxygen Electrocatalysis. Adv. Mater. 2017, 29, 1606207. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, K.; Yurchenko, O.; Melke, J.; Fischer, A.; Urban, G. High electrocatalytic activity of metal-free and non-doped hierarchical carbon nanowalls towards oxygen reduction reaction. Electrochim. Acta 2018, 269, 657–667. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, F.; Zhu, M.; Ma, C.; Zhao, D.; Wang, C.; Zhou, A.; Dai, B.; Ji, J.; Guo, X. N-Doping of plasma exfoliated graphene oxide via dielectric barrier discharge plasma treatment for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2011–2017. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ran, J.; Qiao, S.-Z. Scalable Self-Supported Graphene Foam for High-Performance Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 41980–41987. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Q.; Dai, L. Highly Efficient Metal-Free Growth of Nitrogen-Doped Single-Walled Carbon Nanotubes on Plasma-Etched Substrates for Oxygen Reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef]
- Du, Z.; Wang, S.; Kong, C.; Deng, Q.; Wang, G.; Liang, C.; Tang, H. Microwave plasma synthesized nitrogen-doped carbon nanotubes for oxygen reduction. J. Solid State Electrochem. 2015, 19, 1541–1549. [Google Scholar] [CrossRef]
- Subramanian, P.; Cohen, A.; Teblum, E.; Nessim, G.D.; Bormasheko, E.; Schechter, A. Electrocatalytic activity of nitrogen plasma treated vertically aligned carbon nanotube carpets towards oxygen reduction reaction. Electrochem. Commun. 2014, 49, 42–46. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Ubnoske, S.; Brennaman, M.K.; Song, N.; House, R.L.; Glass, J.T.; Meyer, T.J. Polyethylenimine-Enhanced Electrocatalytic Reduction of CO2 to Formate at Nitrogen-Doped Carbon Nanomaterials. J. Am. Chem. Soc. 2014, 136, 7845–7848. [Google Scholar] [CrossRef] [PubMed]
- Li, O.L.; Wada, Y.; Kaneko, A.; Lee, H.; Ishizaki, T. Oxygen Reduction Reaction Activity of Thermally Tailored Nitrogen-Doped Carbon Electrocatalysts Prepared through Plasma Synthesis. ChemElectroChem 2018, 5, 1995–2001. [Google Scholar] [CrossRef]
- Hyun, K.; Ueno, T.; Li, O.L.; Saito, N. Synthesis of heteroatom-carbon nanosheets by solution plasma processing using N-methyl-2-pyrrolidone as precursor. RSC Adv. 2016, 6, 6990–6996. [Google Scholar] [CrossRef]
- Li, O.L.; Chiba, S.; Wada, Y.; Panomsuwan, G.; Ishizaki, T. Synthesis of graphitic-N and amino-N in nitrogen-doped carbon via a solution plasma process and exploration of their synergic effect for advanced oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 2073–2082. [Google Scholar] [CrossRef]
- Li, O.L.; Chiba, S.; Wada, Y.; Lee, H.; Ishizaki, T. Selective nitrogen bonding states in nitrogen-doped carbon via a solution plasma process for advanced oxygen reduction reaction. RSC Adv. 2016, 6, 109354–109360. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Chiba, S.; Kaneko, Y.; Saito, N.; Ishizaki, T. In situ solution plasma synthesis of nitrogen-doped carbon nanoparticles as metal-free electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 18677–18686. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Electrocatalytic oxygen reduction on nitrogen-doped carbon nanoparticles derived from cyano-aromatic molecules via a solution plasma approach. Carbon 2016, 98, 411–420. [Google Scholar] [CrossRef]
- Morishita, T.; Ueno, T.; Panomsuwan, G.; Hieda, J.; Yoshida, A.; Bratescu, M.A.; Saito, N. Fastest Formation Routes of Nanocarbons in Solution Plasma Processes. Sci. Rep. 2016, 6, 36880. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, T.; Chiba, S.; Kaneko, Y.; Panomsuwan, G. Electrocatalytic activity for the oxygen reduction reaction of oxygen-containing nanocarbon synthesized by solution plasma. J. Mater. Chem. A 2014, 2, 10589–10598. [Google Scholar] [CrossRef]
- Kondratowicz, I.; Nadolska, M.; Şahin, S.; Łapiński, M.; Prześniak-Welenc, M.; Sawczak, M.; Yu, E.H.; Sadowski, W.; Żelechowska, K. Tailoring properties of reduced graphene oxide by oxygen plasma treatment. Appl. Surf. Sci. 2018, 440, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.H.A.; Sofer, Z.; Klímová, K.; Pumera, M. Microwave Exfoliation of Graphite Oxides in H2S Plasma for the Synthesis of Sulfur-Doped Graphenes as Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2016, 8, 31849–31855. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wei, Z.; Wang, X.; Peng, S.; Zhang, X.; Liu, W.-m. Plasma-etched, S-doped graphene for effective hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 4184–4192. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, F.; Chen, Y.; Wang, Z.; Zheng, B.; Wang, X. CVD-grown three-dimensional sulfur-doped graphene as a binder-free electrocatalytic electrode for highly effective and stable hydrogen evolution reaction. J. Mater. Sci. 2018, 53, 7767–7777. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Electrocatalytic oxygen reduction activity of boron-doped carbon nanoparticles synthesized via solution plasma process. Electrochem. Commun. 2015, 59, 81–85. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, M.; Li, C.; Sun, D.; Lei, Y.; Yang, B. Preparation of a porous boron-doped diamond/Ta electrode for the electrocatalytic degradation of organic pollutants. Carbon 2018, 129, 543–551. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Simple one-step synthesis of fluorine-doped carbon nanoparticles as potential alternative metal-free electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9972–9981. [Google Scholar] [CrossRef]
- Tian, Y.; Mei, R.; Xue, D.-Z.; Zhang, X.; Peng, W. Enhanced electrocatalytic hydrogen evolution in graphene via defect engineering and heteroatoms co-doping. Electrochim. Acta 2016, 219, 781–789. [Google Scholar] [CrossRef]
- Lee, S.; Heo, Y.; Bratescu, M.A.; Ueno, T.; Saito, N. Solution plasma synthesis of a boron–carbon–nitrogen catalyst with a controllable bond structure. Phys. Chem. Chem. Phys. 2017, 19, 15264–15272. [Google Scholar] [CrossRef]














| Reaction Type | Samples | Methods | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| MOR | Pt/TiO2 | PEALD | the MOR current density drops to a small value after 1500 s with NCALD < 30. | [15] |
| Pt/C | CAPD | The calculated ECSAs of 75.4 m2/g | [16] | |
| Pt/GNT | H2 plasma | The current density of 97.9 mA/mg and mass activity of 691.1 mA/mg Pt | [20] | |
| Pt/CNTs-HP | H2 plasma | The current density of 15.8 mA/mg | [21] | |
| Au@Pt | Ar plasma | Mass activity up to 48 ± 3 m2/g | [23] | |
| Pt-Ag | SPS | The calculated ECSAs of 28.15 m2/g | [27] | |
| Pt40Pd60 | SPS | The calculated ECSAs of 28.15 m2/g | [28] | |
| Pt69Pd31 | SPS | The catalytic activity of 6.81 mA/cm2 | [29] | |
| PtPd/KB-2 | SPS | The electrocatalytic activity was more than 4 times of that of commercial Pt/C | [30] | |
| Pt/CoPt/MWCNS | SPS | Mass activities of 1719 mA/mg Pt | [32] | |
| Pt/C/TiO2-2 | SPS | Mass activities of 315.2 mA/mg Pt | [33] | |
| Pt-Ru/OCNT | O2 plasma | The onset potential of 0.3 V | [44] | |
| Pt/ZnO/KB | SPS | Catalytic activity of 964 mA/mgPt | [48] | |
| OER | Pt-Ir/TiC | Ar plasma | The current density of 2.5 mA/cm2 at 1 V | [22] |
| PtOaPdOb@Ti3C2Tx | SPS | Activation potential of 1.5 V in 0.1 M KOH | [40] | |
| Ag/1400-15 | Spark plasma sintering | The value of 61 mV | [41] | |
| CoNPs@ C | MPECVD | Overpotential of 270 mV | [50] | |
| CoO–N/C | Cold plasma deposition | The oxygen evolution potential of 378 mV | [55] | |
| Co3O4 nanosheets | Ar plasma | The current density of 44.44 mA/cm2 at 1.6 V | [60] | |
| Vo-COOH | Ar plasma | The low overpotential of 262 mV at 10 mA/cm2 | [61] | |
| Co3O4−x | Ar plasma | The overpotential of 330 mV and Tafel slope of 58 mV/dec | [62] | |
| N-Co3O4 | N2 plasma | The required potential of 1.54 V to reach the current density of 10 mA cm−2 | [64] | |
| P-Co3O4 | Ar plasma | The overpotential of 280 mV and Tafel slope of 51.6 mV/dec | [67] | |
| CoOx-ZIF | O2 plasma | The required potential of 1.548 V to reach the current density of 10 mA cm−2 | [69] | |
| SnCoFe-Ar | Ar plasma | The overpotential of 300 mV and Tafel slope of 42.3 mV/dec | [71] | |
| SrTi0.5Fe0.5O3−y | HT-PVD using O2 plasma | The onset potential of 1.40 at 100 μA | [72] | |
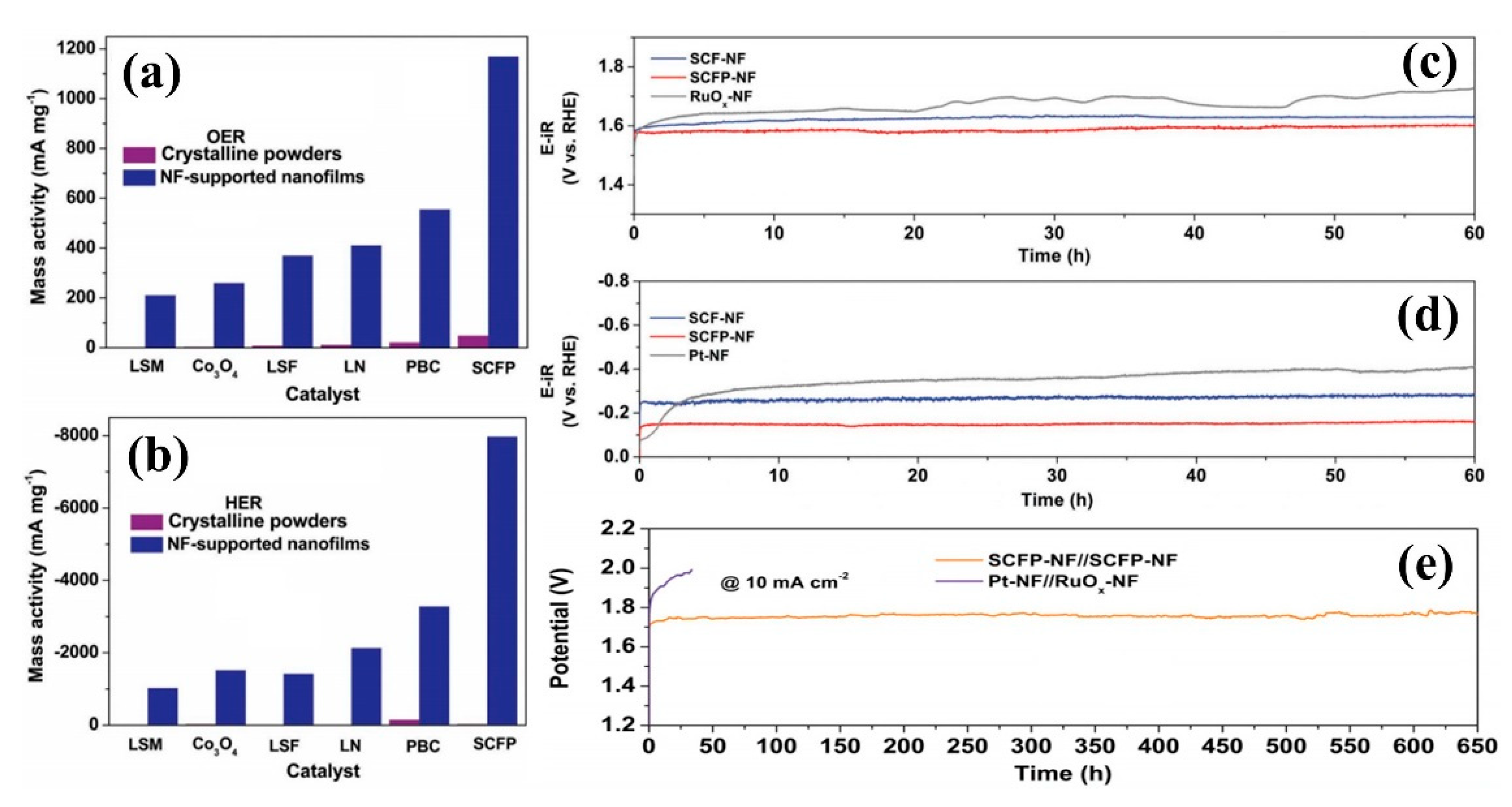
| SCFP-NF | High-energy argon plasma sputtering | The ultra high mass activity of 1000 mA/mg | [73] | |
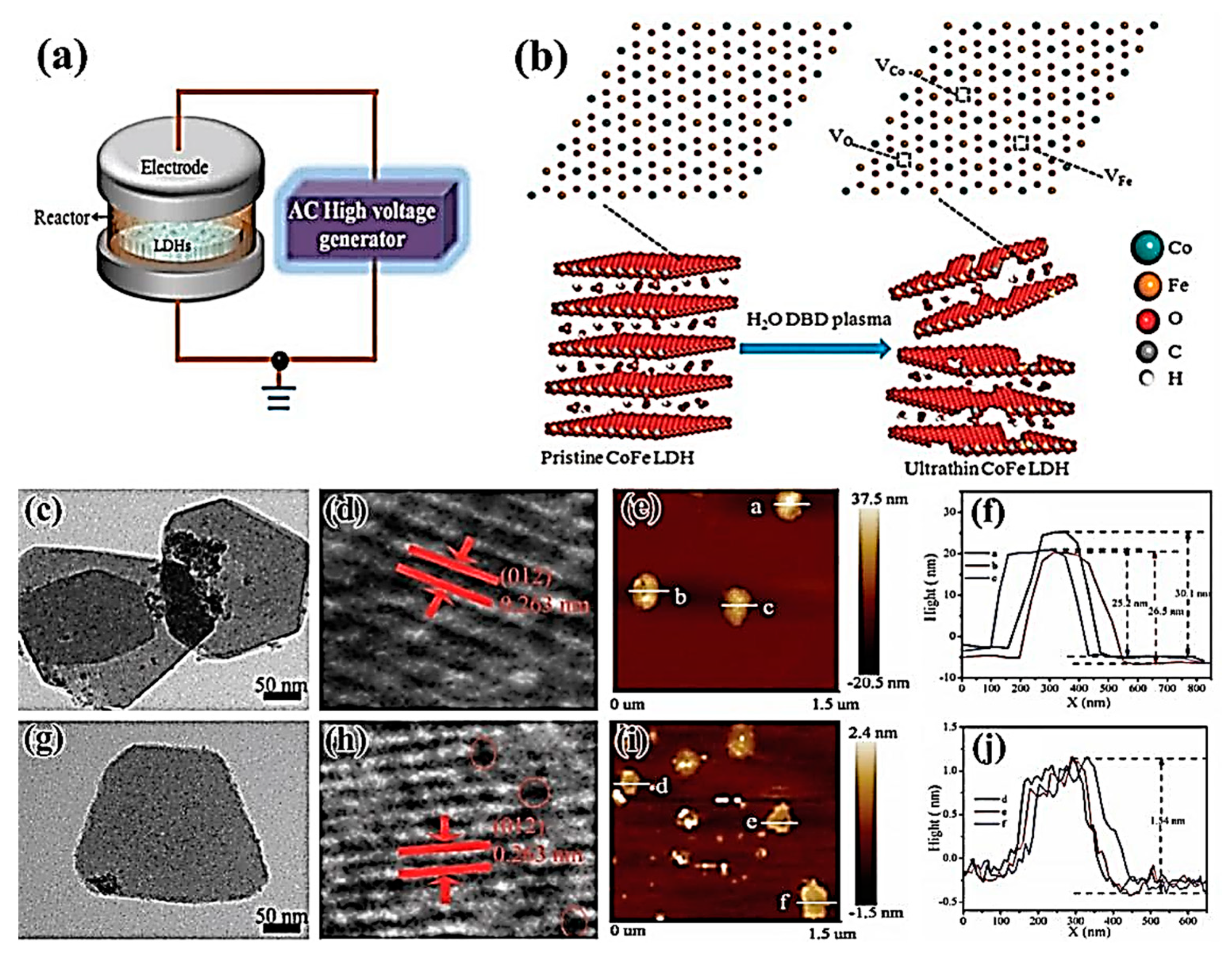
| CoFe-LDHs-H2O | H2O plasma | The overpotential of only 232 mV | [74] | |
| CoFe-LDHs-Ar | Ar plasma | The overpotential of only 232 mV at a current density of 10 mA/cm2 | [77] | |
| CoFe-LDHs-N2 | N2 plasma | The overpotential of only 233 mV at a current density of 10 mA/cm2 | [78] | |
| FeOx/C | pulsed arc plasma deposition | The discharge specific capacity of 500 mAh/g under 100 mA/g | [80] | |
| Co9S8/G | NH3 plasma | The Tafel slope of 82.7 mV/dec | [91] | |
| Cu2S/CF | active iodine DBD plasma | The overpotential of 290 mV at a current density of 10 mA/cm2 | [93] | |
| CoS/Ni3S2-FeS/PNFF | Air plasma | The overpotential of 136 mV at a current density of 10 mA/cm2 | [96] | |
| hNi3N | N2 plasma | The overpotential of 325 mV at a current density of 10 mA/cm2 | [101] | |
| NiCoP | PH3 Plasma | The overpotential of 280 mV at a current density of 10 mA/cm2 | [108] | |
| NiFePi/P | PECVD | The overpotential of 230 mV at a current density of 10 mA/cm2 | [109] | |
| O3-V10-Ni2P | O2 plasam | The Tafel slope of 43.5 mV/dec | [110] | |
| PA-CoPO | H2 plasma | The overpotential of 240 mV and Tafel slope of 53 mV/dec | [113] | |
| Co-PBA-plasma 2 h | Air plasma | The overpotential of 330 mV at a current density of 100 mA/cm2 | [117] | |
| NGF-CFP | N2 plasma | The current density of 10 mA/cm2 at 2.16 V | [128] | |
| SO2OR | Pt3Pd2 and PtPd4 | Plasma sputtering | The lower onset potential of 0.587 ± 0.004 V | [18] |
| HOR | Pt/C | SPS | The ECSAs value of 266 cm2/mg | [38] |
| AMXOR | Ti4O7 | Plasma deposition | A quick oxidation of 0.1 mM AMX | [79] |
| ORR | Pt/C | CAPD | The half-wave potential of 0.87 V | [16] |
| Pt-Ir/TiC | Ar plasma | Nyquist plots of 0.6 V | [22] | |
| Au@Pt | Ar plasma | The peak potential of 0.75 V | [23] | |
| Pt/XC72 | SPS | The onset potential of 1.04 V | [31] | |
| PdAu/KB | SPS | The reduction peak disappeared after about 700 cycles | [34] | |
| AgNW/C | SPS | The high electron density of 13.7 × 10 − 22 m−3 | [37] | |
| Pt/rGO-N | SPS | The half-wave potential of 0.87 V | [39] | |
| Ag/1400-15 | spark plasma sintering | The electron transfer number of 3.9 | [41] | |
| PtNW/GDL | N2 + H2 plasma | The power density of 64 mW/cm2 | [47] | |
| CoO–N/C | Cold plasma deposition | A 2-electron process producing H2O2 | [55] | |
| Ag@Co3O4 | SPS | The half-wave potential of 0.799 V | [56] | |
| 15Co3O4/N-AP/800 | microwave-induced plasma | The Tafel slope of 42 mV/dec | [57] | |
| Co-La-Pt | DC arc discharge plasma | The specific capacity of 3250.2 mAh/g and energy density of 8574.2 Wh/kg at 0.025 mA/cm2 | [58] | |
| Co3O4−x | Ar plasma | The half-wave potential of 0.84 V | [62] | |
| FeOx/C | Pulsed arc plasma deposition | The discharge specific capacity of 500 mAh/g under 100 mA/g | [80] | |
| MnOx@C-D | Air plasma | The electron transfer number of 3.81 | [81] | |
| A-MnO2 | Ar plasma | The power density of 159 mW/cm2 at a current density of 157 mA/cm2 | [82] | |
| Cu3N200/C | PEALD | The half-wave potential of 0.684 V | [103] | |
| Fe-N-CNP-CN | SPS | The onset potential of −0.10 V | [104] | |
| Fe–N/C | Air plasma | The onset potential of 0.88 V at a loading of 0.60 mg/cm2 | [105] | |
| P-Graphene | Ar plasma | The onset potential of 0.912 V and half-wave potential of 0.737 V | [124] | |
| P-CNTs | Ar plasma | The onset potential of 0.83 V | [124] | |
| P-CC | Ar Plasma | The exchange current density of 2.57 × 10−9 A/cm2 | [125] | |
| hCNW-60 | PECVD | The onset potential of 830 mV in 0.1 KOH | [126] | |
| N-PEGO | DBD plasma | The onset potential of 0.89 V | [127] | |
| NCNTs | MPCVD | The onset potential of 0.87 V and the electron transfer number of 4.1 | [130] | |
| VA-NCNTs | N2 plasma | The ORR peak at the potentials of about −0.3 V | [131] | |
| NCNPs | SPS | The onset potential of −0.17 V | [137] | |
| NCNP-3 | SPS | The onset potential of −0.143 V | [138] | |
| BZ90 + DO10 | SPS | The samples were held at 0.5 V with a rotation speed of 1500 rpm for 45,000 s in an O2-saturated 0.1 M KOH solution | [140] | |
| O-rGO | O2 Plasma | The current density of 10 µA/cm2 | [141] | |
| BCNP | SPS | The current densities of 3.15 mA/cm2 at −0.60 V | [145] | |
| FCNP-4 | SPS | The onset potential of 0.22 V and limiting current density of 2.76 mA/cm2 at 0.6 V | [147] | |
| BCN nanocarbon | SPS | 15.1% current decrease after 20000 s | [149] | |
| CO2RR | Au island | O2 plasma | The faradaic efficiency over 95% | [24] |
| CNT/Cu | O2 plasma | Carbon monoxide yields of 178 mmol cm2 mA−1 h−1 | [51] | |
| Cu foil | O2 plasma | The ethylene selectivity of 60% | [52] | |
| Cu nanocube | O2 plasma | The ethylene selectivity of 60% | [53] | |
| ZnO | H2 plasma | The current density of −16.1 mA/cm2 and faradaic efficiency of 83% | [84] | |
| PEI-NCNT/GC | NH3 plasma | The current density of 2.2 mA/cm2 | [132] | |
| HER | Ni–Fe–C | CH4+H2 Plasma carburizing | The activation potential of 57 mV at a current density of 10 mA/cm2 | [49] |
| CoNPs@ C | MPECVD | The overpotential of 153 mV at a current density of 10 mA/cm2 | [50] | |
| P-Co3O4 | Ar plasma | The overpotential of 120 mV and Tafel slope of 52 mV/dec | [67] | |
| SCFP-NF | high-energy argon plasma sputtering | The onset potential of −0.01 V and Tafel slope of 94 mV/dec | [73] | |
| C-30s | C plasma | The Tafel slope of 44 mV/dec | [83] | |
| TaS2-15 min | O2 plasma | The onset potential of 310 mV | [85] | |
| O2-MoS2 | O2 plasma | The current density of 16.3 mA/cm2 at −350 mV | [86] | |
| MoS2 | O2 plasma | The overpotential of 131 mV at a current density of 10 mA/cm2 | [87] | |
| H3Mo12O40P/MoS2 | O2 plasma | The Tafel slope of 44 mV/dec | [88] | |
| MoS2-15 min | H2 plasma | The overpotential of 240 mV at a current density of 10 mA/cm2 | [89] | |
| MoS1.7 | H2 plasma | The overpotential of 143 mV at a current density of 10 mA/cm2 | [90] | |
| Co3S4 PNSvac | Ar plasma | The mass activity of 1056.6 A/g at an overpotential of 200 mV | [92] | |
| WS2 | PEALD | The overpotential of 90 mV at a current density of 100 mA/cm2 | [94] | |
| WS2 | SF6/C4F8 plasma-etched | The overpotential of 100 mV and Tafel slope of 50 mV/dec | [95] | |
| CoS/Ni3S2-FeS/PNFF | Air plasma | The overpotential of 75 mV at a current density of 10 mA/cm2 | [96] | |
| N-MoSe2/VG | MPECVD | The onset potential of 45 mV and overpotential of 98 mV at 10 mA/cm2 | [98] | |
| MoSe2/Mo | N2/H2 plasma | The Tafel slope of 34.7 mV/dec | [99] | |
| Ni3N1−x/NF | Microwave plasma | The overpotential of 55 mV and Tafel slope of 54 mV/dec at a current density of 10 mA/cm2 | [102] | |
| NiCoP | PH3 plasma | The overpotential of 32 mV at a current density of −10 mA/cm2 | [108] | |
| O3-V10-Ni2P | O2 plasma | The overpotential of 108 mV and Tafel slope of 72.3 mV/dec | [110] | |
| Ni-FeP/TiN/CC | Plasma-implanted method | The overpotential of 75 mV at a current density of 10 mA/cm2 | [111] | |
| CoPx | PEALD | The exchange current density of −8.9 × 105 A/cm2 | [112] | |
| PA-CoPO | H2 plasma | The overpotential of 50 mV at a current density of 10 mA/cm2 | [113] | |
| WC nanowalls | DC-PACVD | The Tafel slope of 67 mV/dec | [116] | |
| Co-PBA-plasma 2 h | Air plasma | The overpotential of 77 mV at a current density of 20 mA/cm2 | [117] | |
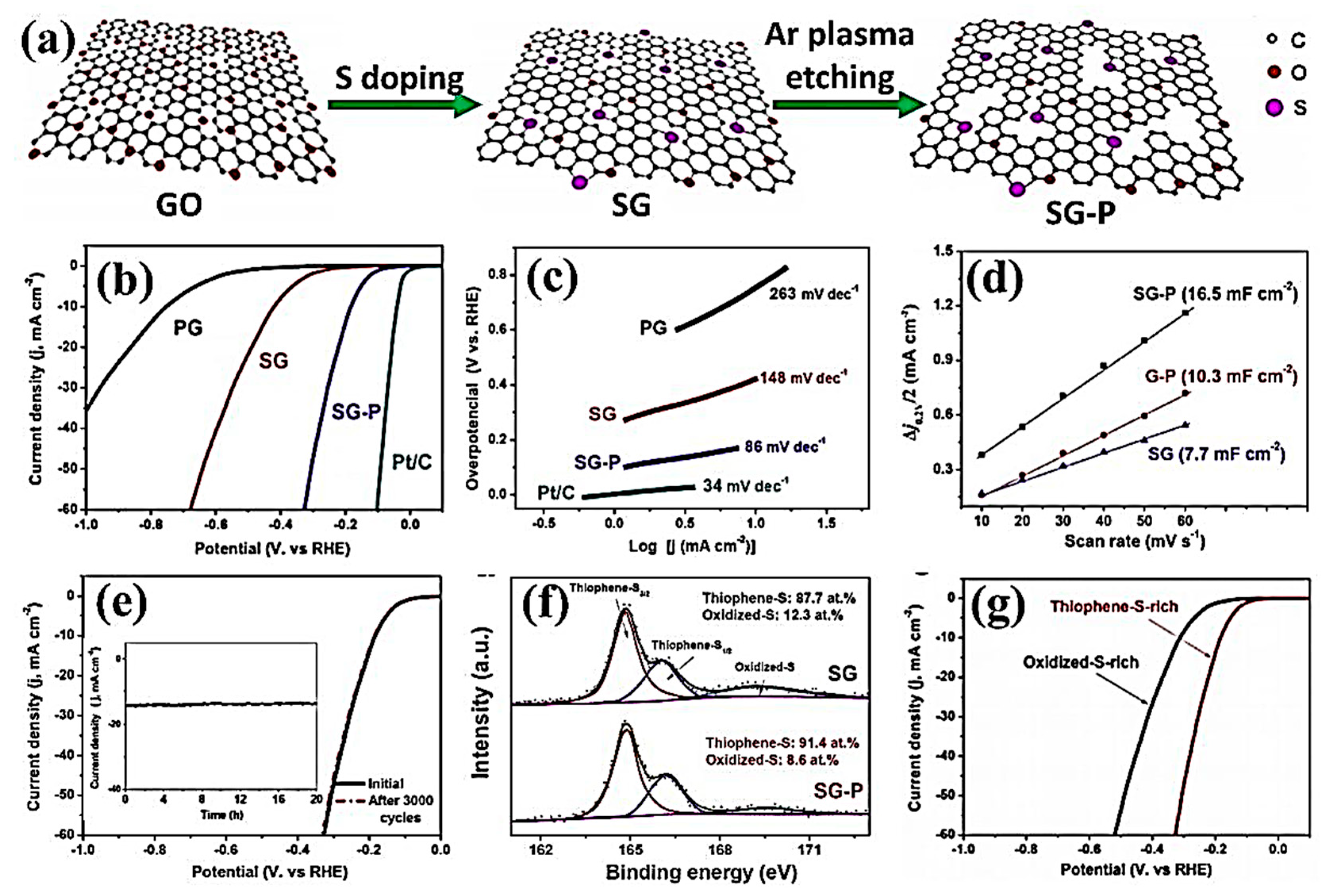
| SG-P | Ar plasma | The overpotential of 178 mV at a current density of 10 mA/cm2 | [143] | |
| 3DSG-Ar | Ar plasma | The Tafel slope of 64 mV/dec | [144] | |
| P-NSG | Ar plasma | The onset potential of 58 mV | [148] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Liu, M.; Ma, C.; Di, L.; Dai, B.; Zhang, L. A Review on the Promising Plasma-Assisted Preparation of Electrocatalysts. Nanomaterials 2019, 9, 1436. https://doi.org/10.3390/nano9101436
Yu F, Liu M, Ma C, Di L, Dai B, Zhang L. A Review on the Promising Plasma-Assisted Preparation of Electrocatalysts. Nanomaterials. 2019; 9(10):1436. https://doi.org/10.3390/nano9101436
Chicago/Turabian StyleYu, Feng, Mincong Liu, Cunhua Ma, Lanbo Di, Bin Dai, and Lili Zhang. 2019. "A Review on the Promising Plasma-Assisted Preparation of Electrocatalysts" Nanomaterials 9, no. 10: 1436. https://doi.org/10.3390/nano9101436





