Bacterial Compatibility/Toxicity of Biogenic Silica (b-SiO2) Nanoparticles Synthesized from Biomass Rice Husk Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of b-SiO2 Nanopowder
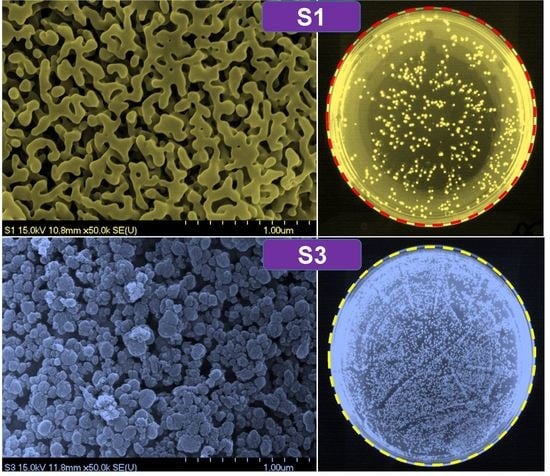
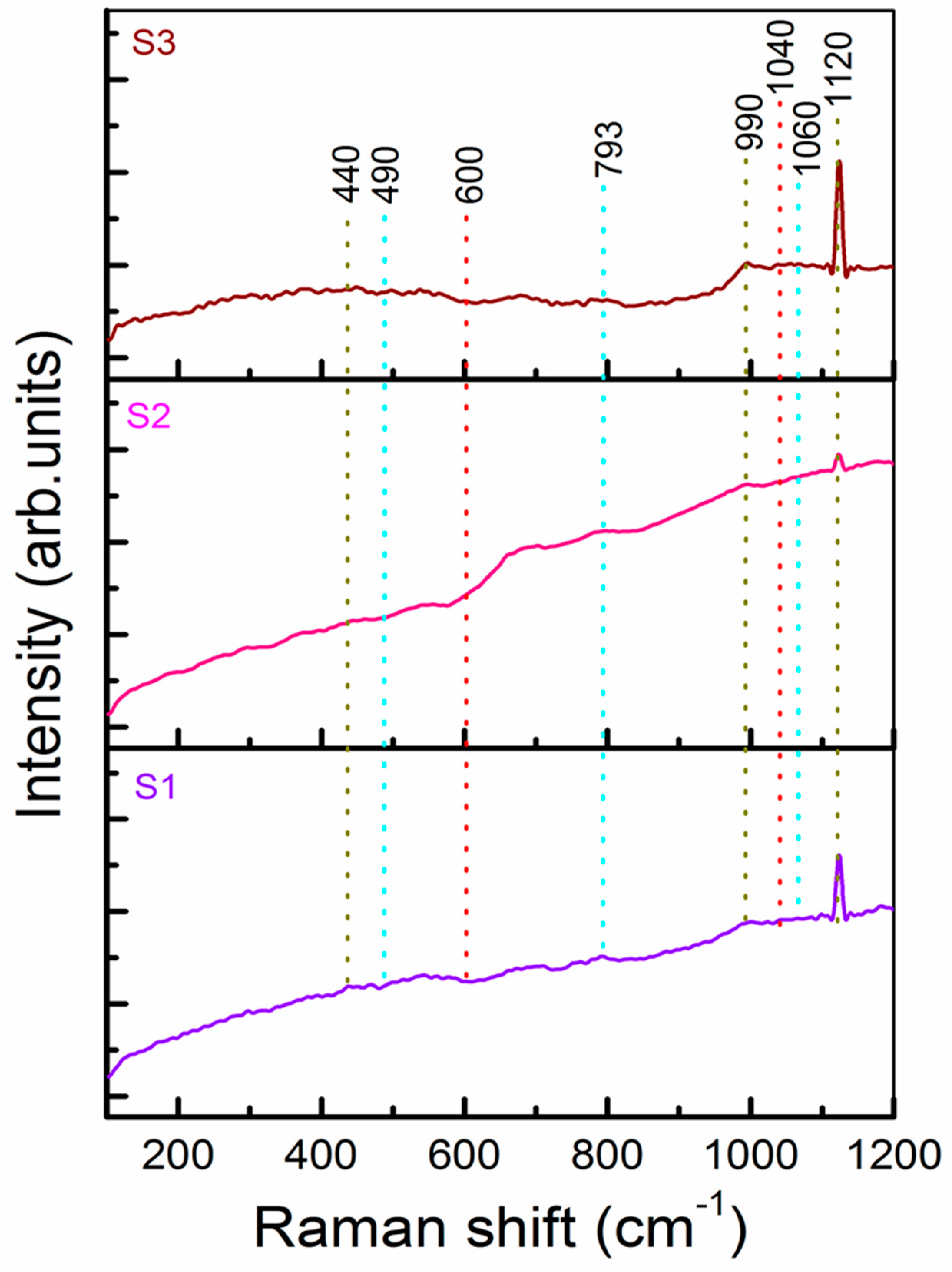
2.3. Characterization of the b-SiO2 Nanopowders: SEM, TEM, XRD, BET, XPS, EDAX, and Raman Spectroscopy
2.4. Bacterial Compatibility/Toxicity Evaluation
2.5. Quantification of the Adhered Bacteria to the b-SiO2 Nanopowders
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.; Ding, X.; Chen, X.; Ma, Y.; Wang, Z.; Zhao, X. A new method of utilizing rice husk: Consecutively preparing d-xylose, organosolv lignin, ethanol and amorphous superfine silica. J. Hazard. Mater. 2015, 291, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Talbot, E.; Pratibha Nalini, R.; Gourbilleau, F.; Pareige, P. Phase transformation in SiOx/SiO2 multilayers for optoelectronics and microelectronics applications. Ultramicroscopy 2013, 132, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, M.; Czarnobaj, K.; Szewczyk, A.; Sawicki, W. Preparation and in vitro characterisation of bioactive mesoporous silica microparticles for drug delivery applications. Mater. Sci. Eng. C 2016, 60, 7–18. [Google Scholar] [CrossRef]
- Garrone, R.; Simpson, T.L.; Pottu-Boumendil, J. Ultrastructure and Deposition of Silica in Sponges. In Silicon and Siliceous Structures in Biological Systems; Simpson, T., Volcani, B., Eds.; Springer: New York, NY, USA, 1981; pp. 495–525. [Google Scholar] [CrossRef]
- Bovee, E.C. Distribution and Forms of Siliceous Structures Among Protozoa. In Silicon and Siliceous Structures in Biological Systems; Simpson, T., Volcani, B., Eds.; Springer: New York, NY, USA, 1981; pp. 233–279. [Google Scholar] [CrossRef]
- Sangster, A.G.; Parry, D.W. Ultrastructure of Silica Deposits in Higher Plants. In Silicon and Siliceous Structures in Biological Systems; Simpson, T., Volcani, B., Eds.; Springer: New York, NY, USA, 1981; pp. 383–407. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alhazmi, M.; Alatiah, K.A.; Alshatwi, A.A. Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram. Int. 2015, 41, 275–281. [Google Scholar] [CrossRef]
- Azam, F.; Volcani, B.E. Germanium-Silicon Interactions in Biological Systems. In Silicon and Siliceous Structures in Biological Systems; Simpson, T., Volcani, B., Eds.; Springer: New York, NY, USA, 1981; pp. 43–67. [Google Scholar] [CrossRef]
- Yu, X.; Tian, J.; Xie, H.; Shen, H.; Wang, Q. The integrated production of microbial lipids and bio-SiO2 from rice husks by an organic electrolytes pretreatment technology. Bioresour. Technol. 2014, 153, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Larichev, Y.V.; Yeletsky, P.M.; Yakovlev, V.A. Study of silica templates in the rice husk and the carbon–silica nanocomposites produced from rice husk. J. Phys. Chem. Solids 2015, 87, 58–63. [Google Scholar] [CrossRef]
- Kumar Rajanna, S.; Vinjamur, M.; Mukhopadhyay, M. Mechanism for formation of Hollow and Granular Silica Aerogel Microspheres from rice husk ash for drug delivery. J. Non Cryst. Solids 2015, 429, 226–231. [Google Scholar] [CrossRef]
- Le, H.T.; Siewert, K.; Ludwig, H.-M. Alkali silica reaction in mortar formulated from self-compacting high performance concrete containing rice husk ash. Constr. Build. Mater. 2015, 88, 10–19. [Google Scholar] [CrossRef]
- Yalçin, N.; Sevinç, V. Studies on silica obtained from rice husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Wang, W.; Martin, J.C.; Fan, X.; Han, A.; Luo, Z.; Sun, L. Silica Nanoparticles and Frameworks from Rice Husk Biomass. ACS Appl. Mater. Interfaces 2012, 4, 977–981. [Google Scholar] [CrossRef]
- Wooldridge, M.S.; Torek, P.V.; Donovan, M.T.; Hall, D.L.; Miller, T.A.; Palmer, T.R.; Schrock, C.R. An experimental investigation of gas-phase combustion synthesis of SiO2 nanoparticles using a multi-element diffusion flame burner. Combust. Flame 2002, 131, 98–109. [Google Scholar] [CrossRef]
- Corradi, A.B.; Bondioli, F.; Ferrari, A.M.; Focher, B.; Leonelli, C. Synthesis of silica nanoparticles in a continuous-flow microwave reactor. Powder Technol. 2006, 167, 45–48. [Google Scholar] [CrossRef]
- Chang, H.; Park, J.-H.; Jang, H.D. Flame synthesis of silica nanoparticles by adopting two-fluid nozzle spray. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 140–144. [Google Scholar] [CrossRef]
- Huang, S.; Jing, S.; Wang, J.; Wang, Z.; Jin, Y. Silica white obtained from rice husk in a fluidized bed. Powder Technol. 2001, 117, 232–238. [Google Scholar] [CrossRef]
- Mochidzuki, K.; Sakoda, A.; Suzuki, M.; Izumi, J.; Tomonaga, N. Structural Behavior of Rice Husk Silica in Pressurized Hot-Water Treatment Processes. Ind. Eng. Chem. Res. 2001, 40, 5705–5709. [Google Scholar] [CrossRef]
- Jal, P.K.; Sudarshan, M.; Saha, A.; Patel, S.; Mishra, B.K. Synthesis and characterization of nanosilica prepared by precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 173–178. [Google Scholar] [CrossRef]
- Liou, T.-H. Preparation and characterization of nano-structured silica from rice husk. Mater. Sci. Eng. A 2004, 364, 313–323. [Google Scholar] [CrossRef]
- Carmona, V.B.; Oliveira, R.M.; Silva, W.T.L.; Mattoso, L.H.C.; Marconcini, J.M. Nanosilica from rice husk: Extraction and characterization. Ind. Crop. Prod. 2013, 43, 291–296. [Google Scholar] [CrossRef]
- Capeletti, L.B.; de Oliveira, L.F.; Gonçalves, K.D.A.; de Oliveira, J.F.A.; Saito, Â.; Kobarg, J.; Santos, J.H.Z.D.; Cardoso, M.B. Tailored Silica–Antibiotic Nanoparticles: Overcoming Bacterial Resistance with Low Cytotoxicity. Langmuir 2014, 30, 7456–7464. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Athinarayanan, J.; Periasamy, V.S. Biocompatibility assessment of rice husk-derived biogenic silica nanoparticles for biomedical applications. Mater. Sci. Eng. C 2015, 47, 8–16. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Kinnari, T.J.; Esteban, J.; Gomez-Barrena, E.; Zamora, N.; Fernandez-Roblas, R.; Nieto, A.; Doadrio, J.C.; López-Noriega, A.; Ruiz-Hernández, E.; Arcos, D.; et al. Bacterial adherence to SiO2-based multifunctional bioceramics. J. Biomed. Mater. Res. A 2009, 89, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Le, V.H.; Thuc, C.N.H.; Thuc, H.H. Synthesis of silica nanoparticles from Vietnamese rice husk by sol-gel method. Nanoscale Res. Lett. 2013, 8, 58. [Google Scholar] [CrossRef]
- Gnana Kumar, G.; Karunagaran, B.; Nahm, K.; Nimma Elizabeth, R. Nanometer Sized Silver Particles Embedded Silica Particles—Spray Method. Nanoscale Res. Lett. 2009, 4, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.S.; Kanyal, S.S.; Madaan, N.; Vail, M.A.; Dadson, A.E.; Engelhard, M.H.; Linford, M.R. Silicon (100)/SiO2 by XPS. Surf. Sci. Spectra 2013, 20, 36–42. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Jiang, L.; Wang, W.; Wu, D.; Zhan, J.; Wang, Q.; Wu, Z.; Jin, R. Preparation of silver quantum dots embedded water-soluble silica/PAAc hybrid nanoparticles and their bactericidal activity. Mater. Chem. Phys. 2007, 104, 230–234. [Google Scholar] [CrossRef]
- Liou, T.-H.; Yang, C.-C. Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Mater. Sci. Eng. B 2011, 176, 521–529. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Rahman, I.A.; Sipaut, C.S. Optical properties of amorphous organo-modified silica nanoparticles produced via co-condensation method. Ceram. Int. 2010, 36, 333–338. [Google Scholar] [CrossRef]
- Umari, P.; Alfredo, P. First-principles analysis of the Raman spectrum of vitreous silica: Comparison with the vibrational density of states. J. Phys. Condens. Matter 2003, 15, S1547. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mammone, J.F.; Nicol, M.F. Raman investigation of ring configurations in vitreous silica. Nature 1981, 292, 140–141. [Google Scholar] [CrossRef]
- Chiodini, N.; Meinardi, F.; Morazzoni, F.; Paleari, A.; Scotti, R.; Spinolo, G. Tin doped silica by sol–gel method: Doping effects on the SiO2 Raman spectrum. Solid State Commun. 1998, 109, 145–150. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.K.; Sharma, A.R.; Pamidimarri, S.D.V.N.; Gaur, J.; Singh, B.P.; Sekar, S.; Kim, D.Y.; Lee, S.S. Bacterial Compatibility/Toxicity of Biogenic Silica (b-SiO2) Nanoparticles Synthesized from Biomass Rice Husk Ash. Nanomaterials 2019, 9, 1440. https://doi.org/10.3390/nano9101440
Sharma SK, Sharma AR, Pamidimarri SDVN, Gaur J, Singh BP, Sekar S, Kim DY, Lee SS. Bacterial Compatibility/Toxicity of Biogenic Silica (b-SiO2) Nanoparticles Synthesized from Biomass Rice Husk Ash. Nanomaterials. 2019; 9(10):1440. https://doi.org/10.3390/nano9101440
Chicago/Turabian StyleSharma, Sanjeev K., Ashish R. Sharma, Sudheer D. V. N. Pamidimarri, Jyotshana Gaur, Beer Pal Singh, Sankar Sekar, Deuk Young Kim, and Sang Soo Lee. 2019. "Bacterial Compatibility/Toxicity of Biogenic Silica (b-SiO2) Nanoparticles Synthesized from Biomass Rice Husk Ash" Nanomaterials 9, no. 10: 1440. https://doi.org/10.3390/nano9101440
APA StyleSharma, S. K., Sharma, A. R., Pamidimarri, S. D. V. N., Gaur, J., Singh, B. P., Sekar, S., Kim, D. Y., & Lee, S. S. (2019). Bacterial Compatibility/Toxicity of Biogenic Silica (b-SiO2) Nanoparticles Synthesized from Biomass Rice Husk Ash. Nanomaterials, 9(10), 1440. https://doi.org/10.3390/nano9101440