Fabrication of Robust Hydrogen Evolution Reaction Electrocatalyst Using Ag2Se by Vacuum Evaporation
Abstract
:1. Introduction
2. Materials and Methods
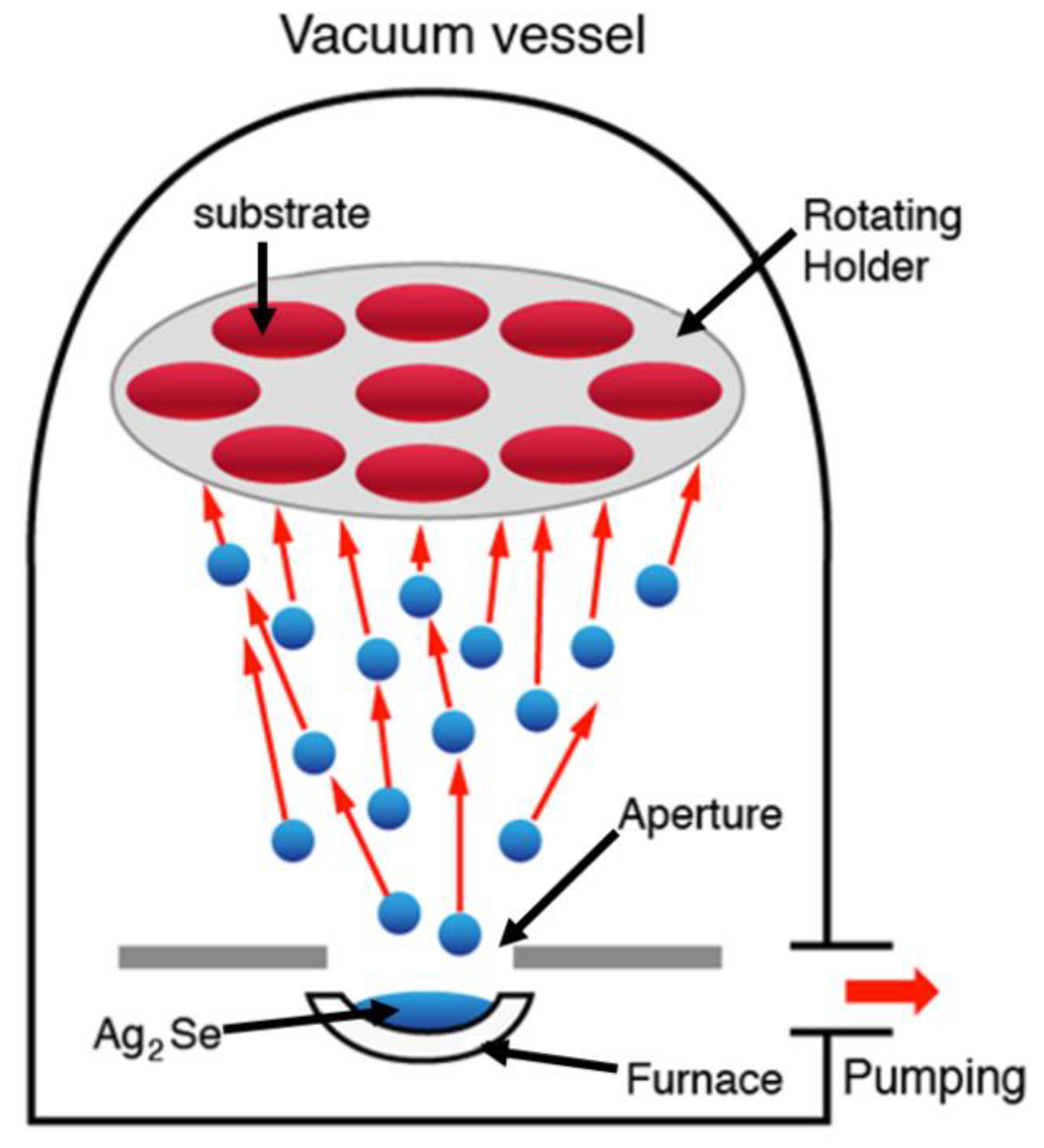
2.1. Material Synthesis
2.2. Electrochemical Measurements
2.3. Characterizations
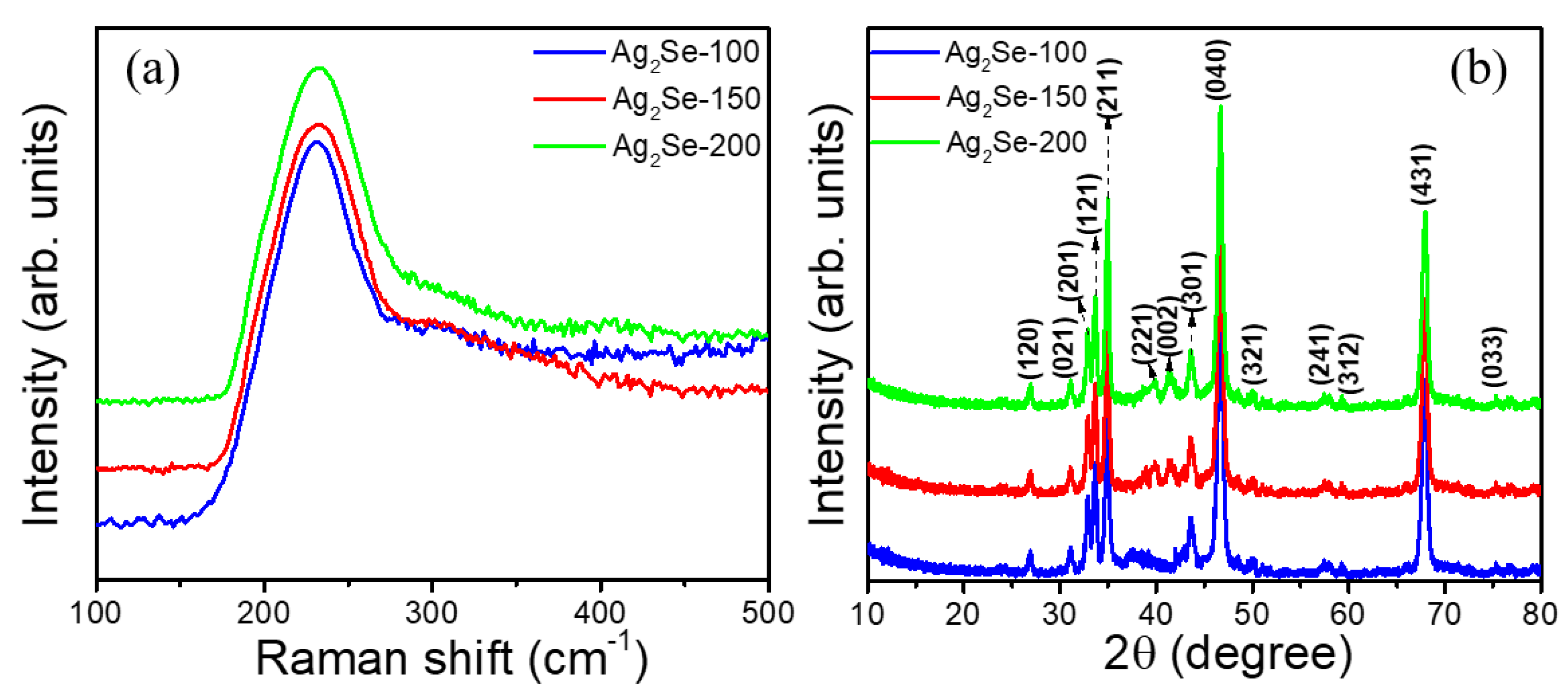
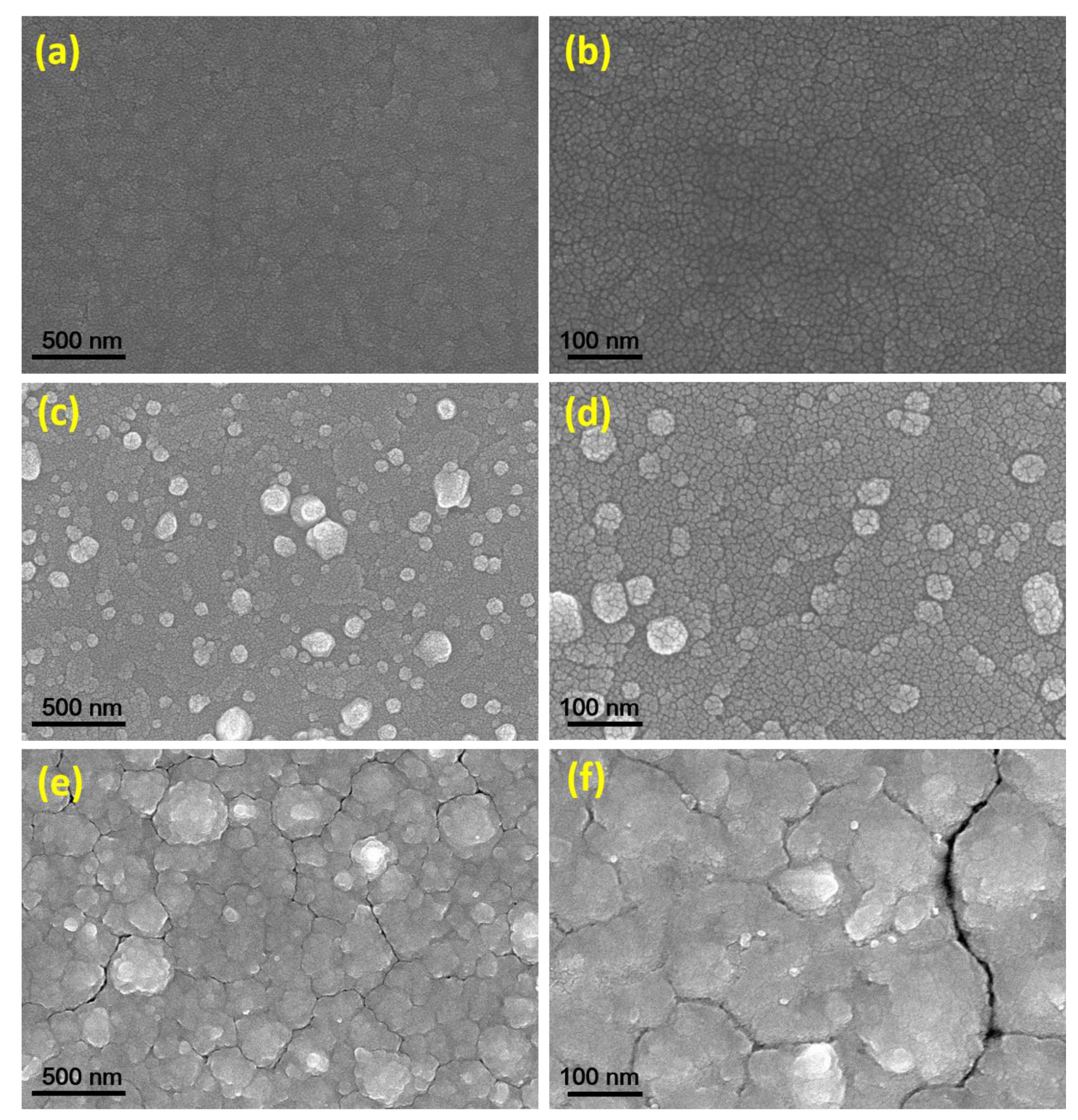
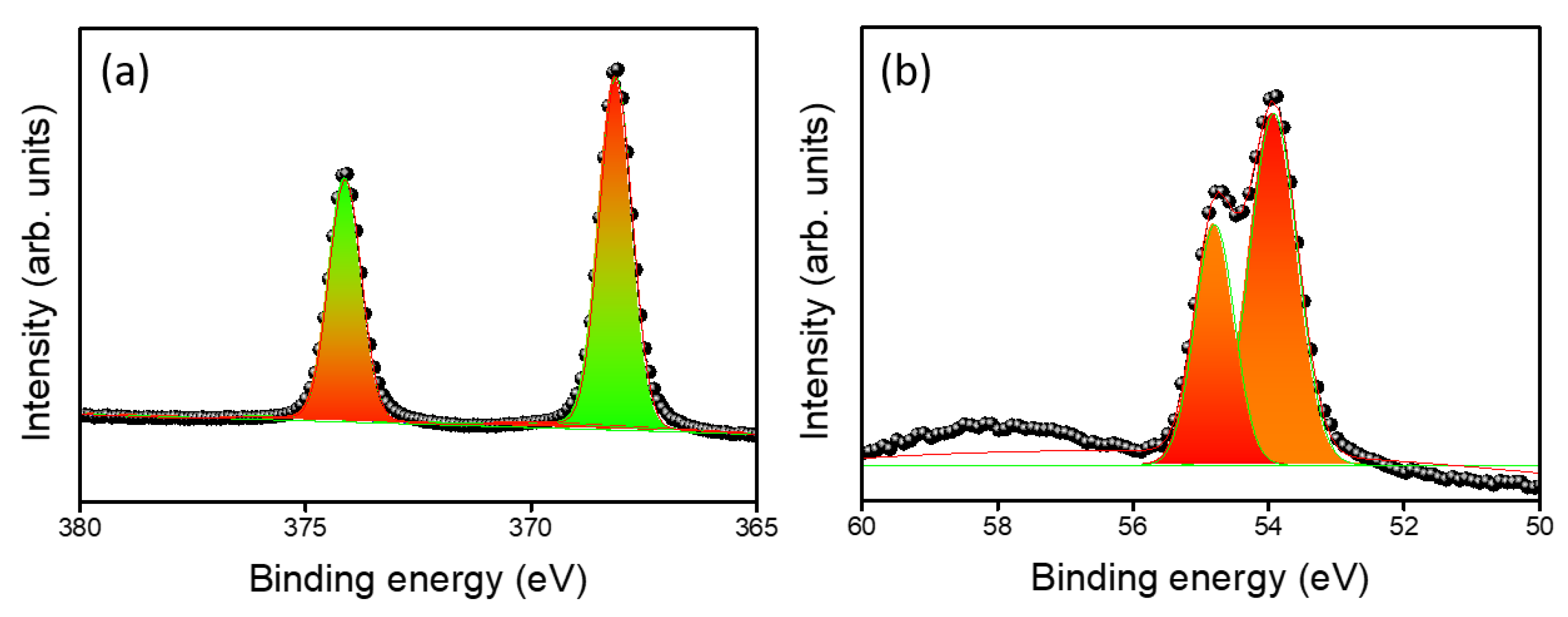
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Elvington, M.; Brown, J.; Arachchige, S.M.; Brewer, K.J. Photocatalytic hydrogen production from water employing a Ru, Rh, Ru molecular device for photoinitiated electron collection. J. Am. Chem. Soc. 2007, 129, 10644–10645. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Schneider, J.; Jarosz, P.; Eisenberg, R. Photocatalytic generation of hydrogen from water using a platinum (ii) terpyridyl acetylide chromophore. J. Am. Chem. Soc. 2006, 128, 7726–7727. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, Y.; Pan, J.; Zhong, Z.; Xu, R. Self-assembled dye–layered double hydroxide–Pt nanoparticles: A novel H2 evolution system with remarkably enhanced stability. Nanoscale 2011, 3, 4655–4661. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ozawa, H. Homogeneous catalysis of platinum (ii) complexes in photochemical hydrogen production from water. Coord. Chem. Rev. 2007, 251, 2753–2766. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Akbar, K.; Naqvi, B.A.; Abbas, S.M.; Kim, H.-S.; Chun, S.-H.; Jung, J. Fabrication of MoSe2 decorated three-dimensional graphene composites structure as a highly stable electrocatalyst for improved hydrogen evolution reaction. Renew. Energy 2019, 143, 1659–1669. [Google Scholar] [CrossRef]
- Vikraman, D.; Akbar, K.; Hussain, S.; Yoo, G.; Jang, J.Y.; Chun, S.H.; Jung, J.; Park, H.J. Direct synthesis of thickness-tunable MoS2 quantum dot thin layers: Optical, structural and electrical properties and their application to hydrogen evolution. Nano Energy 2017, 35, 101–114. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Karuppasamy, K.; Chun, S.H.; Jung, J.; Kim, H.S. Design of basal plane edges in metal-doped nanostripes-structured MoSe2 atomic layers to enhance hydrogen evolution reaction activity. ACS Sustain. Chem. Eng. 2019, 7, 458–469. [Google Scholar] [CrossRef]
- Huang, J.-F.; Chang, W.-R. Cu(I)-mediating Pt reduction to form Pt-nanoparticle-embedded Nafion composites and their electrocatalytic O2 reduction. J. Mater. Chem. 2012, 22, 17961–17966. [Google Scholar] [CrossRef]
- Chen, H.-H.; Huang, J.-F. EDTA assisted highly selective detection of As3+ on au nanoparticle modified glassy carbon electrodes: Facile in situ electrochemical characterization of Au nanoparticles. Anal. Chem. 2014, 86, 12406–12413. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Huang, Y.; Sun, J.; Zhu, Z.; Nielsen, R.J.; He, R.; Bao, J.; Goddard, W.A., III; Chen, S. Efficient hydrogen evolution by ternary molybdenum sulfoselenide particles on self-standing porous nickel diselenide foam. Nat. Commun. 2016, 7, 12765. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ju, P.; Li, W.; Zhao, Y.; Han, X. Ag2S nanoparticle-decorated MoS2 for enhanced electrocatalytic and photoelectrocatalytic activity in water splitting. Dalton Trans. 2017, 46, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Nazir, R.; Mahala, C.; Fageria, P.; Chaudhary, S.; Gangopadhyay, S.; Pande, S. Ag2S/Ag heterostructure: A promising electrocatalyst for the hydrogen evolution reaction. Langmuir 2017, 33, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, L. Controlled Synthesis of Ag2S, Ag2Se, and Ag Nanofibers by Using a General Sacrificial Template and Their Application in Electronic Device Fabrication. Adv. Funct. Mater. 2008, 18, 1249–1256. [Google Scholar] [CrossRef]
- Ren, H.; Xu, W.; Zhu, S.; Cui, Z.; Yang, X.; Inoue, A. Synthesis and properties of nanoporous Ag2S/CuS catalyst for hydrogen evolution reaction. Electrochim. Acta 2016, 190, 221–228. [Google Scholar] [CrossRef]
- Mendoza-Galvan, A.; García-García, E.; Vorobiev, Y.; Gonzalez-Hernandez, J. Structural, optical and electrical characterization of amorphous SexTe1−x thin film alloys. Microelectron. Eng. 2000, 51, 677–687. [Google Scholar] [CrossRef]
- Pandiaraman, M.; Soundararajan, N. Micro-raman studies on thermally evaporated Ag2Se thin films. J. Theor. Appl. Phys. 2012, 6, 7. [Google Scholar] [CrossRef]
- Romand, M.; Roubin, M.; Deloume, J. ESCA studies of some copper and silver selenides. J. Electron Spectrosc. Relat. Phenom. 1978, 13, 229–242. [Google Scholar] [CrossRef]
- Yang, L.; Fu, Q.; Wang, W.; Huang, J.; Huang, J.; Zhang, J.; Xiang, B. Large-area synthesis of monolayered MoS2(1−x) Se2x with a tunable band gap and its enhanced electrochemical catalytic activity. Nanoscale 2015, 7, 10490–10497. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhang, J.; Li, H.; Tang, Z.; Wang, X. Nanosheet-assembled MoSe2 and S-doped MoSe2−x nanostructures for superior lithium storage properties and hydrogen evolution reactions. Inorg. Chem. Front. 2015, 2, 931–937. [Google Scholar] [CrossRef]
- Velazquez, J.M.; Saadi, F.H.; Pieterick, A.P.; Spurgeon, J.M.; Soriaga, M.P.; Brunschwig, B.S.; Lewis, N.S. Synthesis and hydrogen-evolution activity of tungsten selenide thin films deposited on tungsten foils. J. Electroanal. Chem. 2014, 716, 45–48. [Google Scholar] [CrossRef]
- Xu, X.; Du, P.; Chen, Z.; Huang, M. An electrodeposited cobalt–selenide-based film as an efficient bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 2016, 4, 10933–10939. [Google Scholar] [CrossRef]
- Masud, J.; Swesi, A.T.; Liyanage, W.P.; Nath, M. Cobalt selenide nanostructures: An efficient bifunctional catalyst with high current density at low coverage. ACS Appl. Mater. Interfaces 2016, 8, 17292–17302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, X.; Hedhili, M.N.; Huang, K.-W.; Li, L.-J.; Zhang, W. Symmetrical synergy of hybrid CoS2-WS2 electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 15552–15558. [Google Scholar] [CrossRef]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: An efficient 3d electrode for overall water splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Truong, L.; Kathalingam, A.; Chun, S.H.; Jung, J.; Park, H.J.; Kim, H.S. Improved hydrogen evolution reaction performance using MoS2-WS2 heterostructures by physicochemical process. ACS Sustain. Chem. Eng. 2018, 6, 8400–8409. [Google Scholar] [CrossRef]
- Gao, M.-R.; Lin, Z.-Y.; Zhuang, T.-T.; Jiang, J.; Xu, Y.-F.; Zheng, Y.-R.; Yu, S.-H. Mixed-solution synthesis of sea urchin-like nise nanofiber assemblies as economical Pt-free catalysts for electrochemical H2 production. J. Mater. Chem. 2012, 22, 13662–13668. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Zhao, H.; Du, Y. Colloidally synthesized MoSe2/graphene hybrid nanostructures as efficient electrocatalysts for hydrogen evolution. J. Mater. Chem. A 2015, 3, 19706–19710. [Google Scholar] [CrossRef]
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-controllable WS2(1–x)Se2x nanotubes for efficient hydrogen evolution reaction. ACS Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef]
- Zhao, B.; Huang, J.; Fu, Q.; Yang, L.; Zhang, J.; Xiang, B. MoS2/NbSe2 hybrid nanobelts for enhanced hydrogen evolution. J. Electrochem. Soc. 2016, 163, H384–H387. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Wang, F.; Shifa, T.A.; Cheng, Z.; Wang, Z.; Xu, K.; Zhan, X.; Wang, Q.; Huang, Y. Enhanced electrochemical H2 evolution by few-layered metallic WS2(1−x) Se2x nanoribbons. Adv. Funct. Mater. 2015, 25, 6077–6083. [Google Scholar] [CrossRef]
- Shifa, T.A.; Wang, F.; Liu, K.; Cheng, Z.; Xu, K.; Wang, Z.; Zhan, X.; Jiang, C.; He, J. Efficient catalysis of hydrogen evolution reaction from WS2(1−x) P2x nanoribbons. Small 2017, 13, 1603706. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Akbar, K.; Adaikalam, K.; Lee, S.H.; Chun, S.H.; Jung, J.; Kim, H.S.; Park, H.J. Facile synthesis of molybdenum diselenide layers for high-performance hydrogen evolution electrocatalysts. ACS Omega 2018, 3, 5799–5807. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Akbar, K.; Vikraman, D.; Karuppasamy, K.; Kim, H.S.; Chun, S.H.; Jung, J. Synthesis of mos2(1−x)Se2x and WS2(1−x)Se2x alloys for enhanced hydrogen evolution reaction performance. Inorg. Chem. Front. 2017, 4, 2068–2074. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.D.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Afzal, R.A.; Song, W.; An, K.S.; Farooq, A.; Park, J.Y.; Chun, S.H.; Jung, J. WS(1−x)Sex nanoparticles decorated three-dimensional graphene on nickel foam: A robust and highly efficient electrocatalyst for the hydrogen evolution reaction. Nanomaterials 2018, 8, 929. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Truong, L.; Karuppasamy, K.; Kim, H.J.; Maiyalagan, T.; Chun, S.H.; Jung, J.; Kim, H.S. Fabrication of MoS2/WSe2 heterostructures as electrocatalyst for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 480, 611–620. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Rameshkumar, S.; Jaiganesh, G.; Jayalakshmi, V.; Palanivel, B. Ab initio calculation of structural stability, electronic and optical properties of Ag2Se. AIP Conf. Proc. 2015, 1665, 090024. [Google Scholar]
- Naumov, P.; Barkalov, O.; Mirhosseini, H.; Felser, C.; Medvedev, S. Atomic and electronic structures evolution of the narrow band gap semiconductor Ag2Se under high pressure. J. Phys. Condens. Matter 2016, 28, 385801. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Foner, S.; Hudson, R. Ionization potential of the OH free radical by mass spectrometry. J. Chem. Phys. 1956, 25, 602–603. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef]
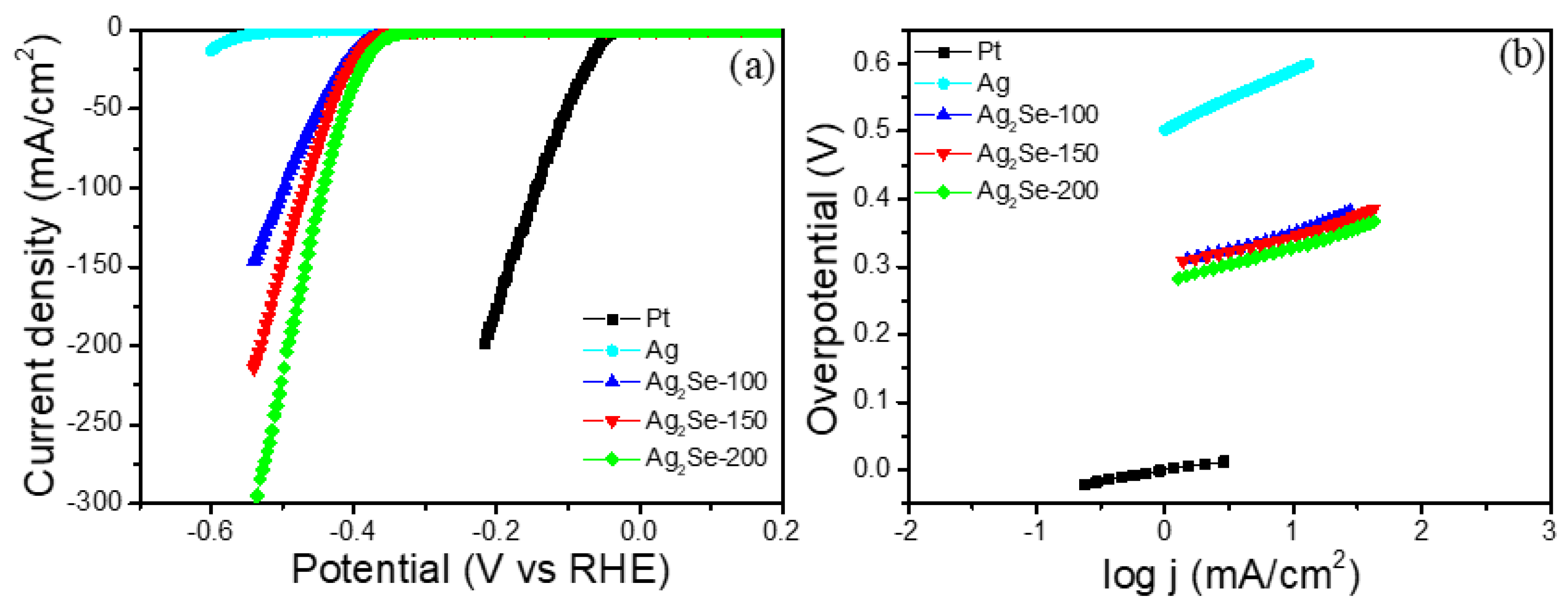
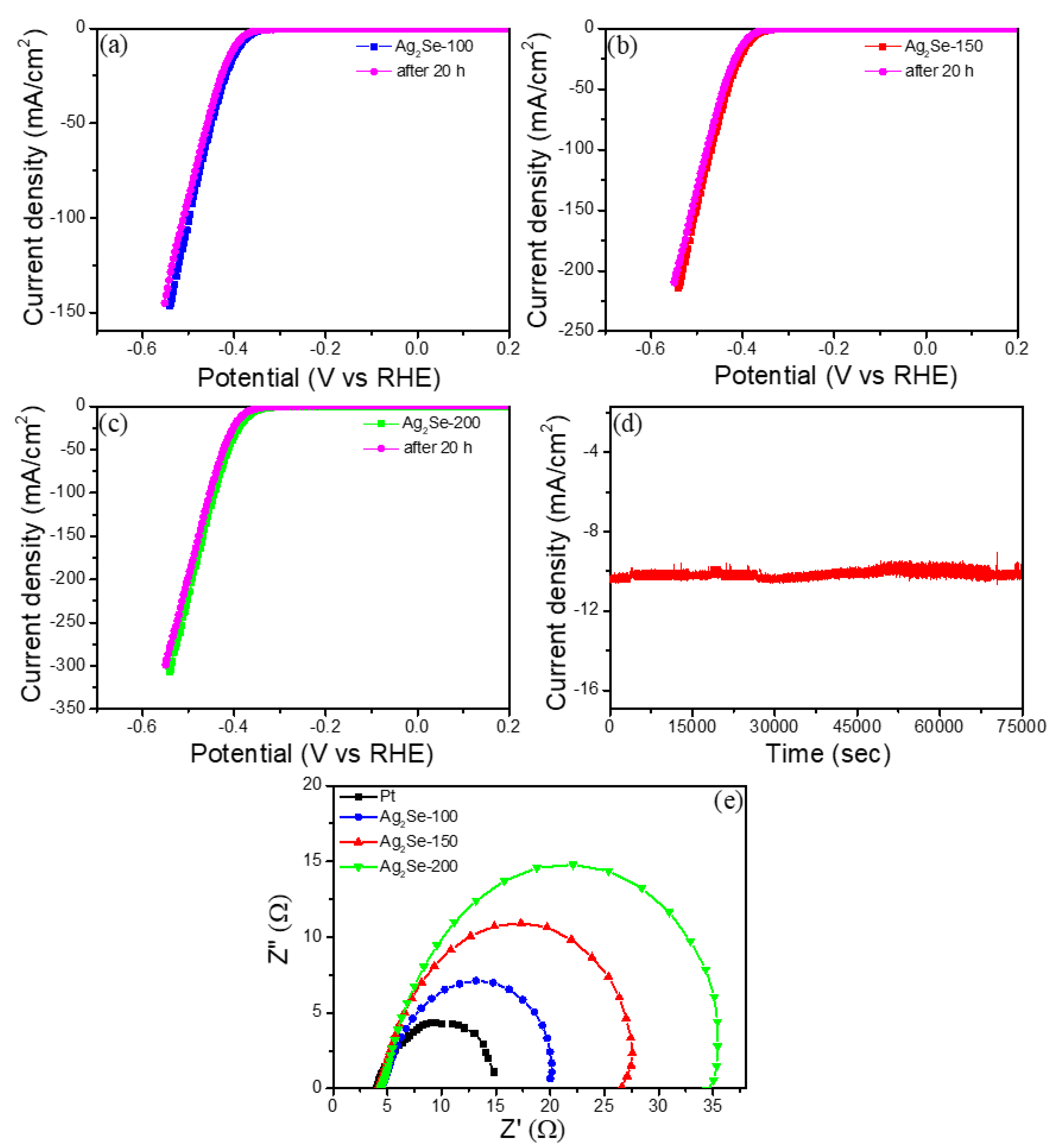

| Sample | Overpotential (mV vs. RHE) at 10 mA·cm−2 | Tafel Slope (mV·dec−1) | Exchange Current Density (j0, mA·cm−2) |
|---|---|---|---|
| Pt | 54 | 31 | 9.86 × 10−1 |
| Ag2Se-200 | 367 | 53 | 1.02 × 10−3 |
| Ag2Se-150 | 382 | 51 | 5.12 × 10−4 |
| Ag2Se-100 | 390 | 55 | 6.45 × 10−4 |
| Ag | 588 | 87 | 1.31 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Chae, J.; Akbar, K.; Vikraman, D.; Truong, L.; Naqvi, B.A.; Abbas, Y.; Kim, H.-S.; Chun, S.-H.; Kim, G.; et al. Fabrication of Robust Hydrogen Evolution Reaction Electrocatalyst Using Ag2Se by Vacuum Evaporation. Nanomaterials 2019, 9, 1460. https://doi.org/10.3390/nano9101460
Hussain S, Chae J, Akbar K, Vikraman D, Truong L, Naqvi BA, Abbas Y, Kim H-S, Chun S-H, Kim G, et al. Fabrication of Robust Hydrogen Evolution Reaction Electrocatalyst Using Ag2Se by Vacuum Evaporation. Nanomaterials. 2019; 9(10):1460. https://doi.org/10.3390/nano9101460
Chicago/Turabian StyleHussain, Sajjad, Jinwoong Chae, Kamran Akbar, Dhanasekaran Vikraman, Linh Truong, Bilal Abbas Naqvi, Yawar Abbas, Hyun-Seok Kim, Seung-Hyun Chun, Gunn Kim, and et al. 2019. "Fabrication of Robust Hydrogen Evolution Reaction Electrocatalyst Using Ag2Se by Vacuum Evaporation" Nanomaterials 9, no. 10: 1460. https://doi.org/10.3390/nano9101460
APA StyleHussain, S., Chae, J., Akbar, K., Vikraman, D., Truong, L., Naqvi, B. A., Abbas, Y., Kim, H.-S., Chun, S.-H., Kim, G., & Jung, J. (2019). Fabrication of Robust Hydrogen Evolution Reaction Electrocatalyst Using Ag2Se by Vacuum Evaporation. Nanomaterials, 9(10), 1460. https://doi.org/10.3390/nano9101460







