Reactivity and Chemical Sintering of Carey Lea Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silver Colloids and Films
2.3. Chemical Treatment of Ag NPs
2.4. Characterization
3. Results and Discussion
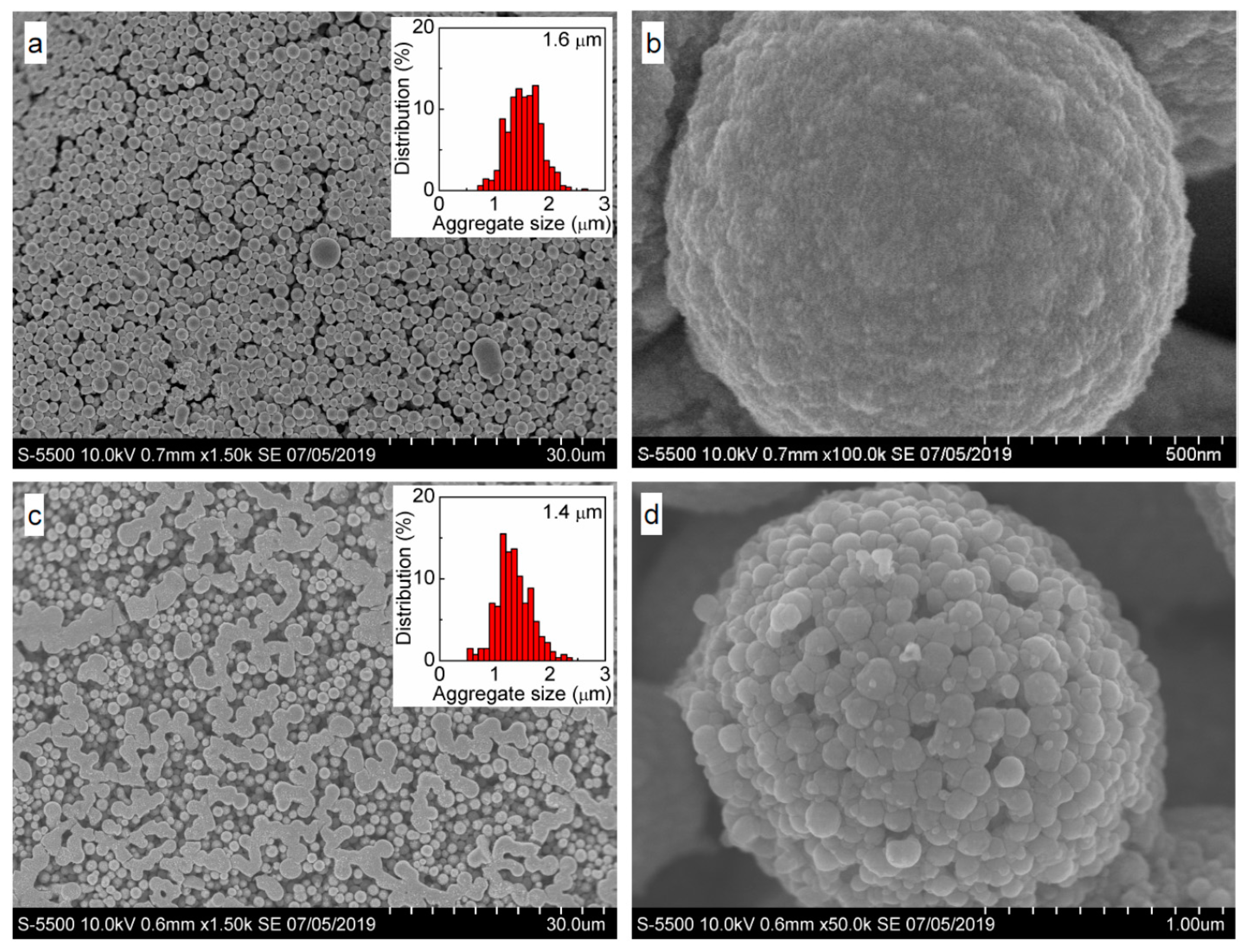
3.1. Intrinsic Ag Nanoparticles
3.2. Sulfidation of Carey Lea Nanoparticles
3.3. Oxidation of Silver Nanoparticles with Hydrogen Peroxide
3.4. Oxidation and Sintering of Ag NPs Film with HAuCl4 Solutions
3.5. Oxidation and Sintering of Ag NP Film with Solutions of H2PtCl6 and H2PdCl4
4. On the Mechanisms Involved
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calderón-Jiménez, B.; Johnson, M.E.; Bustos, A.R.M.; Murphy, K.E.; Winchester, M.R.; Baudrit, V.R. Silver nanoparticles: Technological advances, societal impacts, and metrological challenges. Front. Chem. 2017, 5, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Krutyakov, Y.A.; Kudrinsky, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis of highly stable silver colloids stabilized with water soluble sulfonated polyaniline. Appl. Surf. Sci. 2010, 256, 7037–7042. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for printed electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of colloidal silver nanoparticles: Capping action of citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Carey Lea, M. Allotropic forms of silver. Am. J. Sci. 1889, 37, 476–491. [Google Scholar] [CrossRef]
- Frens, G.; Overbeek, J.T.G. Carey Lea’s colloidal silver. Kolloid-Zeitschrift und Zeitschrift für Polymere 1969, 233, 922–929. [Google Scholar] [CrossRef]
- Jolivet, J.P.; Gzara, M.; Mazieres, J.; Lefebvre, J. Physicochemical study of aggregation in silver colloids. J. Colloid Interface Sci. 1985, 107, 429–441. [Google Scholar] [CrossRef]
- Fornasiero, D.; Grieser, F. The kinetics of electrolyte induced aggregation of Carey Lea silver colloids. J. Colloid Interface Sci. 1991, 141, 168–179. [Google Scholar] [CrossRef]
- Dementeva, O.V.; Malkovskii, A.V.; Filippenko, M.A.; Rudoy, V.M. Comparative study of the properties of silver hydrosols prepared by “citrate” and “citrate–sulfate” procedures. Colloid J. 2008, 70, 561–573. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Zhang, Z.; Yu, J. Bimodal sintered silver nanoparticle paste with ultrahigh thermal conductivity and shear strength for high temperature thermal interface material applications. ACS Appl. Mater. Interf. 2015, 7, 9157–9168. [Google Scholar] [CrossRef] [PubMed]
- Kilin, D.S.; Prezhdo, O.V.; Xia, Y. Shape-controlled synthesis of silver nanoparticles: Ab initio study of preferential surface coordination with citric acid. Chem. Phys. Lett. 2008, 458, 113–116. [Google Scholar] [CrossRef]
- Siiman, O.; Bumm, L.A.; Callaghan, R.; Blatchford, C.O.; Kerker, M. Surface-enhanced Raman scattering by citrate on colloidal silver. J. Phys. Chem. 1983, 87, 1014–1023. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Vorobyev, S.A.; Saikova, S.V.; Vishnyakova, E.A.; Romanchenko, A.S.; Zharkov, S.M.; Larichev, Y.V. On the nature of citrate-derived surface species on Ag nanoparticles: Insights from x-ray photoelectron spectroscopy. Appl. Surf. Sci. 2018, 427, 687–694. [Google Scholar] [CrossRef]
- He, D.; Grag, S.; Waite, T.D. H2O2-mediated oxidation of zero-valent silver and resultant interactions among silver nanoparticles, silver ions, and reactive oxygen species. Langmuir 2012, 28, 10266–10275. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, Y. High chemical reactivity of silver nanoparticles toward hydrochloric acid. J. Colloid Int. Sci. 2006, 303, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Mikhlin, Y.L.; Vishnyakova, E.A.; Romanchenko, A.S.; Saikova, S.V.; Likhatski, M.N.; Larichev, Y.V.; Tuzikov, V.; Zaikovskii, V.I.; Zharkov, S.M. Oxidation of Ag nanoparticles in aqueous media: Effect of particle size and capping. Appl. Surf. Sci. 2014, 297, 75–83. [Google Scholar] [CrossRef]
- Bell, R.A.; Kramer, J.R. Structural chemistry and geochemistry of silver-sulfur compounds: Critical review. Environ. Toxicol. Chem. 1999, 18, 9–22. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Y.; Sullivan, N.; Chen, Y. Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ. Sci. Technol. 2011, 45, 4422–4428. [Google Scholar] [CrossRef]
- Liu, J.; Pennell, K.G.; Hurt, R.H. Kinetics and Mechanisms of Nanosilver Oxysulfidation. Environ. Sci. Technol. 2011, 45, 7345–7353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levard, C.; Reinsch, B.C.; Michel, F.M.; Oumahi, C.; Lowry, G.V.; Brown, G.E. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: Impact on dissolution rate. Environ. Sci. Technol. 2011, 45, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Reinsch, B.C.; Levard, C.; Li, Z.; Ma, R.; Wise, A.; Gregory, K.B.; Brown, G.E., Jr.; Lowry, G.V. Sulfidation of silver nanoparticles decreases Escherichia coli growth inhibition. Environ. Sci. Technol. 2012, 46, 6992–7000. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.-H. Silver nanopartciles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. A simple and “green” method for the synthesis of Au, Ag, and Au-Ag alloy nanoparticles. Green Chem. 2006, 8, 34–38. [Google Scholar] [CrossRef]
- Mallin, M.P.; Murphy, C.J. Solution-Phase Synthesis of Sub-10 nm Au-Ag Alloy Nanoparticles. Nanoletters 2002, 2, 1235–1237. [Google Scholar] [CrossRef]
- Cao, Y.; Jin, R.; Mirkin, C.A. DNA-Modified Core-Shell Ag/Au Nanoparticles. J. Am. Chem. Soc. 2001, 123, 7961–7962. [Google Scholar] [CrossRef]
- Zhang, Q.; Lee, J.Y.; Yang, J.; Boothroyd, C.; Zhang, J. Size and composition tunable Ag-Au alloy nanoparticles by replacement reactions. Nanotechnology 2007, 18, 245605. [Google Scholar] [CrossRef]
- Wang, C.; Peng, S.; Chan, R.; Sun, S. Synthesis of AuAg Alloy Nanoparticles from Core/Shell-Structured Ag/Au. Small 2009, 5, 567–570. [Google Scholar] [CrossRef]
- Pal, A.; Shah, S.; Devi, S. Synthesis of Au, Ag and Au-Ag alloy nanoparticles in aqueous polymer solution. Colloids Surfaces A Physicochem. Eng. Aspects 2007, 302, 51–57. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Liang, J.; Lee, J.Y. Synthesis of monodisperse Ag-Au alloy nanoparticles with independently tunable morphology, composition, size, and surface chemistry and their 3-D superlattices. Adv. Funct. Mater. 2009, 19, 1387–1398. [Google Scholar] [CrossRef]
- Shore, M.S.; Wang, J.; Johnston-Peck, A.C.; Oldenburg, A.L.; Tracy, J.B. Synthesis of Au(Core)/Ag(Shell) nanoparticles and their conversion to AuAg alloy nanoparticles. Small 2011, 7, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Shitaba, T.; Bunker, B.A.; Zhang, Z.; Meisel, D.; Vardeman, C.F.; Gezelter, J.D. Size-dependent spontaneous alloying of Au-Ag nanoparticles. J. Am. Chem. Soc. 2002, 124, 11989–11996. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Lee, J.Y.; Zhang, J.; Boothroyd, C. Synthesis of Ag@AgAu metal core/alloy shell simetallic nanoparticles with tunable shell compositions by a galvanic replacement reaction. Small 2008, 4, 1067–1071. [Google Scholar] [CrossRef]
- Sui, N.; Yue, R.; Wang, Y.; Bai, Q.; An, R.; Xiao, H.; Wang, L.; Liu, M.; Yu, W.W. Boosting methanol oxidation reaction with Au@AgPt yolk-shell nanoparticles. J. Alloys Compd. 2019, 790, 792–798. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H.; Abad, M.Z.Y.; Noroozifar, M. Controlled organization of building blocks to prepare three-dimensional architecture of Pd-Ag aerogel as a high active electrocatalyst toward formic acid oxidation. Compos. Part B Eng. 2019, 172, 309–315. [Google Scholar] [CrossRef]
- Feng, L.; Gao, G.; Huang, P.; Wang, X.; Zhang, C.; Zhang, J.; Guo, S.; Cui, D. Preparation of Pt-Ag alloy nanoisland/graphene hybrid composites and its high stability and catalytic activity in methanol electro-oxidation. Nanoscale Res. Lett. 2011, 6, 551. [Google Scholar] [CrossRef]
- Yousaf, A.B.; Imran, M.; Zeb, A.; Wen, T.; Xie, X.; Jiang, Y.-F. Single phase PtAg bimetallic alloy nanoparticles highly dispersed on reduced graphene oxide for electrocatalytic application of methanol oxidation reaction. Electrochim. Acta 2016, 197, 117–125. [Google Scholar] [CrossRef]
- He, W.; Wu, X.; Liu, J.; Zhang, K.; Chu, W.; Feng, L.; Hu, X.; Zhou, W.; Xie, S. Formation of AgPt alloy nanoislands via chemical etching with tunable optical properties. Langmuir 2009, 26, 4443–4448. [Google Scholar] [CrossRef]
- Machocki, A.; Ioannides, T.; Stasinska, B.; Gac, W.; Avgouropoulos, G.; Delimaris, D.; Grzegorczyk, W.; Pasieczna, S. Manganese–lanthanum oxides modified with silver for the catalytic combustion of methane. J. Catal. 2004, 227, 282–296. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Palyanova, G.A.; Tomashevich, Y.V.; Vishnyakova, E.A.; Vorobyev, S.A.; Kokh, K.A. XPS and Ag L3-edge XANES characterization of silver- and silver-gold sulfoselenides. J. Phys. Chem. Solids 2018, 116, 292–298. [Google Scholar] [CrossRef]
- Baetzold, R.C.; Apai, G.; Shustorovich, E. Surface core-level shifts for Pt single-crystal surfaces. Phys. Rev. B 1982, 26, 4022–4027. [Google Scholar] [CrossRef]
- Butcher, D.R.; Grass, M.E.; Zeng, Z.; Aksoy, F.; Bluhm, H.; Li, W.X.; Mun, B.S.; Somorjai, G.A.; Liu, Z. In Situ Oxidation Study of Pt(110) and Its Interaction with CO. J. Am. Chem. Soc. 2011, 133, 20319–20325. [Google Scholar] [CrossRef] [PubMed]
- Militello, M.C.; Simko, S.J. Elemental Palladium by XPS. Surf. Sci. Spectra 1994, 3, 387–394. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Srabionyan, V.V.; Mikheykina, E.B.; Avakyan, L.A.; Murzin, V.Y.; Zubavichus, Y.V.; Zizak, I.; Guterman, V.E.; Bugaev, L.A. Atomic Structure of Bimetallic Nanoparticles in PtAg/C Catalysts: Determination of Components Distribution in the Range from Disordered Alloys to “Core–Shell” Structures. J. Phys. Chem. C 2015, 119, 3217–3227. [Google Scholar] [CrossRef]
- Gadaud, P.; Caccuri, V.; Bertheau, D.; Carr, J.; Mihlet, X. Ageing sintered silver: Relationship between tensile behavior, mechanical properties and the nanoporous structure evolution. Mater. Sci. Eng. 2016, 669, 379–386. [Google Scholar] [CrossRef]








| Sample | C | Ag | O | S | Au | Pt | Pd | Fe | Cl |
|---|---|---|---|---|---|---|---|---|---|
| Initial silver film | 46.2 | 30.3 | 22.0 | – | – | – | – | 1.5 | – |
| + H2O2 (7 wt. %) | 51.1 | 26.8 | 21.4 | – | – | – | – | 0.7 | – |
| + H2S | 36.9 | 32.1 | 18.5 | 12.5 | – | – | – | – | – |
| + HAuCl4 (4 mM, 12 min) | 41.1 | 38.9 | 5.6 | – | 9.6 | – | – | – | 4.9 |
| + H2PtCl6 (1.0 mM, 20 min) | 67.4 | 21.9 | 2.7 | – | – | 3.8 | – | 0.4 | 3.8 |
| + H2PdCl4 (0.33 mM, 40 min) | 71.3 | 19.9 | 4.8 | – | – | – | 1.6 | – | 2.4 |
| Reaction Time (min) | Concentration (at. %) | |||||
|---|---|---|---|---|---|---|
| C | O | Cl | Ag | Au | ||
| 0.2 mM HAuCl4 | 4 | 34.4 | 14.2 | 10.4 | 38.3 | 0.5 |
| 12 | 45.4 | 7.8 | 12.9 | 33.3 | 0.6 | |
| 30 | 54.4 | 8.5 | 3.6 | 31.7 | 1.2 | |
| 60 | 47.4 | 6.5 | 6.4 | 38.3 | 1.4 | |
| 4 mM HAuCl4 | 12 | 41.1 | 5.6 | 4.9 | 38.9 | 9.6 |
| 60 | 53.5 | 19.7 | 10.0 | 9.0 | 7.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyev, S.; Vishnyakova, E.; Likhatski, M.; Romanchenko, A.; Nemtsev, I.; Mikhlin, Y. Reactivity and Chemical Sintering of Carey Lea Silver Nanoparticles. Nanomaterials 2019, 9, 1525. https://doi.org/10.3390/nano9111525
Vorobyev S, Vishnyakova E, Likhatski M, Romanchenko A, Nemtsev I, Mikhlin Y. Reactivity and Chemical Sintering of Carey Lea Silver Nanoparticles. Nanomaterials. 2019; 9(11):1525. https://doi.org/10.3390/nano9111525
Chicago/Turabian StyleVorobyev, Sergey, Elena Vishnyakova, Maxim Likhatski, Alexander Romanchenko, Ivan Nemtsev, and Yuri Mikhlin. 2019. "Reactivity and Chemical Sintering of Carey Lea Silver Nanoparticles" Nanomaterials 9, no. 11: 1525. https://doi.org/10.3390/nano9111525
APA StyleVorobyev, S., Vishnyakova, E., Likhatski, M., Romanchenko, A., Nemtsev, I., & Mikhlin, Y. (2019). Reactivity and Chemical Sintering of Carey Lea Silver Nanoparticles. Nanomaterials, 9(11), 1525. https://doi.org/10.3390/nano9111525






