A Sulfur Copolymers (SDIB)/Polybenzoxazines (PBz) Polymer Blend for Electrospinning of Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
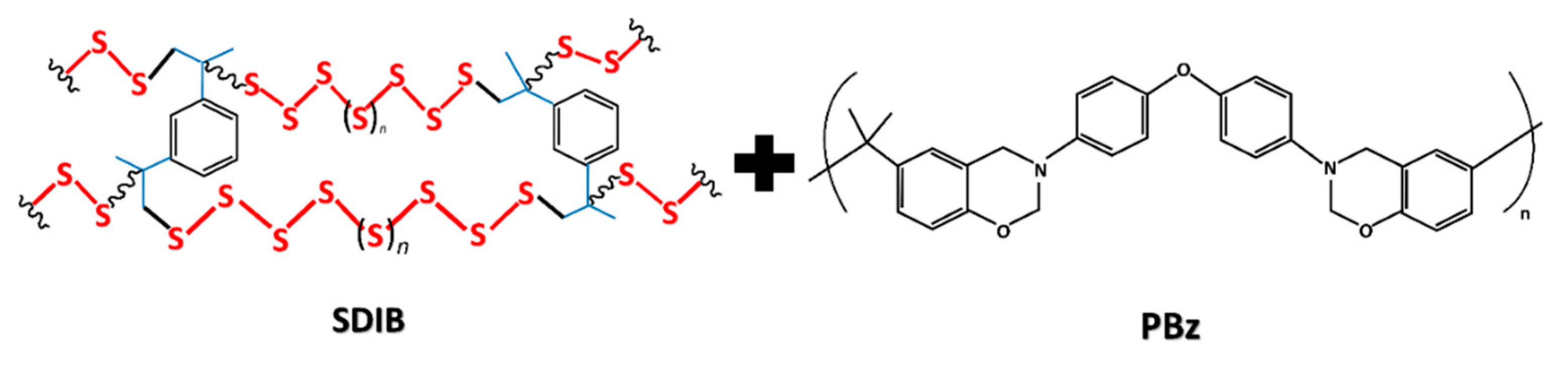
2.2. Synthesis of SDIB via Inverse Vulcanization Process
2.3. Preparation of Polymer Blend
2.4. Electrospinning of Polymer Blend
2.5. Characterization of Electrospun Nanofiber Mat
3. Results and Discussion
3.1. Preparation of Polymer Blend
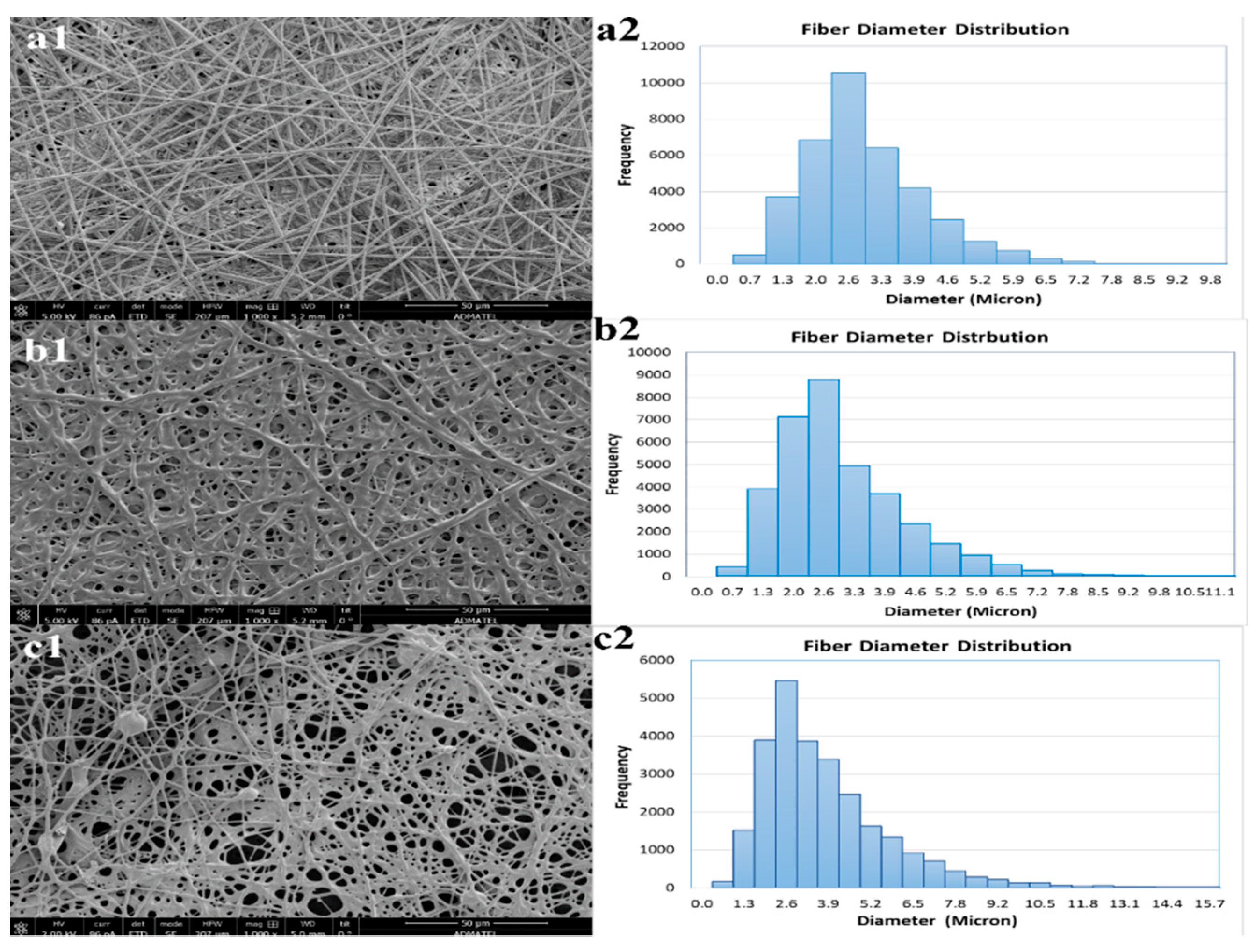
3.2. Electrospinning of Polymer Blend
3.3. SEM-EDX of Electrospun Nanofibers
3.4. FTIR Spectroscopy
3.5. Thermal Property
3.6. Water Contact Angle
3.7. Solubility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Parker, D.J.; Jones, H.A.; Petcher, S.; Cervini, L.; Akhtar, R.; Hasell, T. Low cost and renewable sulfur-polymers by inverse vulcanization, and their potential for mercury capture. J. Mater. Chem. A 2017, 1, 11682–11692. [Google Scholar] [CrossRef]
- Boyd, D.A. Sulfur and its role in modern materials science. Angew. Chem. 2016, 55, 15486–15502. [Google Scholar] [CrossRef]
- Jena, K.K.; Alhassan, S.M. Melt processed elemental sulfur reinforced polyethylene composites. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Moon, J.; Kalb, P.D.; Milian, L.; Northrup, P.A. Characterization of a sustainable sulfur polymer concrete using activated fillers. Cement Concr. Compos. 2016, 67, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Arslan, M.; Kiskan, B.; Yagci, Y. Combining elemental sulfur with polybenzoxazines via inverse vulcanization. Macromolecules 2016, 49, 767–773. [Google Scholar] [CrossRef]
- Penczek, S.; Slazak, R.; Duda, A. Anionic copolymerization of elemental sulfur. Nature 1978, 273, 738–739. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. Anionic copolymerization of elemental sulfur with 2,2-dimethylthiirane. Chem. Macromol. Chem. Phys. 1980, 181, 995–1001. [Google Scholar] [CrossRef]
- Blight, L.B.; Currell, B.R.; Nash, B.J.; Scott, T.M.; Stillo, C. Chemistry of the modification of sulphur by the use of dicyclopentadiene and of styrene. Br. Polym. J. 1980, 12, 5–11. [Google Scholar] [CrossRef]
- Tsuda, T.; Takeda, A. Palladium-catalysed cycloaddition copolymerisation of diynes with elemental sulfur to poly(thiophene)s. Chem. Commun. 1996, 11, 1317–1318. [Google Scholar] [CrossRef]
- Ding, Y.; Hay, A.S. Copolymerization of elemental sulfur with cyclic (arylene disulfide) oligomers. J. Polym. Sci. A 1997, 35, 2961–2968. [Google Scholar] [CrossRef]
- Khawaja, S.Z.; Kumar, S.V.; Jena, K.K.; Alhassan, S.M. Flexible sulfur film from inverse vulcanization technique. Mater. Lett. 2017, 203, 58–61. [Google Scholar] [CrossRef]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Pyun, J. Inverse vulcanization of elemental sulfur to prepare polymeric electrode materials for Li-S batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef]
- Jyotishkumar, P.; Thomas, S.; Grohens, Y. Characterization of Polymer Blends; Wiley-VCH: Weinheim, Germany, 2015; Volume 1, Chapter 1; pp. 1–5. [Google Scholar]
- Thielke, M.W.; Bultema, L.A.; Brauer, D.D.; Richter, B.; Fischer, M.; Theato, P. Rapid Mercury (II) removal by electrospun sulfur copolymers. Polymers 2016, 8, 266. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.F.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.H.D.; Rahman, M.A. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Sun, G.; Wang, M.; Yu, J. Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 2013, 58, 1173–1243. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of nanofibers and their applications for energy devices. J. Nanomater. 2015, 16, 122. [Google Scholar] [CrossRef]
- Du, Q.; Wu, J.; Yang, H. Pt@Nb-TiO2 catalyst membranes fabricated by electrospinning and atomic layer deposition. ACS Catal. 2014, 4, 144–151. [Google Scholar] [CrossRef]
- Miao, Y.; Fan, W.; Chen, D.; Liu, T. High-performance supercapacitors based on hollow polyaniline nanofibers by electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun poly(vinyl alcohol)/glucose oxidase biocomposite membranes for biosensor applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Yanilmaz, M.; Lu, Y.; Dirican, M.; Fu, K.; Zhang, X. Nanoparticle-on-nanofiber hybrid membrane separators for lithium-ion batteries via combining electrospraying and electrospinning techniques. J. Memb. Sci. 2014, 456, 57–65. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Tkachenko, I.M.; Bliznyuk, V.N.; Shevchenko, V.V. Fluorinated polybenzoxazines as advanced phenolic resins for leading-edge applications. React. Funct. Polym. 2018, 133, 71–92. [Google Scholar] [CrossRef]
- Khan, M.M.; Halder, K.; Shishatskiy, S.; Filiz, V. Synthesis and crosslinking of polyether-based main chain benzoxazine polymers and their gas separation performance. Polymers 2018, 10, 221. [Google Scholar] [CrossRef]
- Shen, X.; Dai, J.; Liu, Y.; Liu, X.; Zhu, J. Synthesis of high performance polybenzoxazine networks from bio-based furfurylamine: Furan vs benzene ring. Polymers 2017, 122, 258–269. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, Y.L. Polyelectrolyte composite membranes of polybenzimidazole and crosslinked polybenzimidazole-polybenzoxazine electrospun nanofibers for proton exchange membrane fuel cells. J. Mater. Chem. A 2013, 1, 1171–1178. [Google Scholar] [CrossRef]
- De Meuse, M. High Temperature Polymer Blends; Woodhead Publishing: Cambridge, England, 2014; pp. 1–232. [Google Scholar]
- Khalil, M.; Saeed, S.; Ahmad, Z. Properties of binary polyimide blends containing hexafluoroisopropylidene group. J. Macromol. Sci. Part A Pure Appl. Chem. 2006, 44, 55–63. [Google Scholar] [CrossRef]
- Carraher, C.E. Introduction to Polymer Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 8; pp. 289–293. [Google Scholar]
- Isayev, A.I. Encyclopedia of Polymer Blends; Wiley-VCH: Weinheim, Germany, 2010; Volume 1, pp. 135–140. [Google Scholar]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook, 2nd ed.; Springer Nature: Dordrecht, The Netherlands, 2014; pp. 1–24. [Google Scholar]
- Mazinani, S.; Darvishmanesh, S.; Ramazani, R.; Van Der Bruggen, B. Miscibility of polyimide blends: Physicochemical characterization of two high performance polyimide polymers. REACT 2017, 111, 88–101. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Fundamentals of electrospinning and processing technologies. Part. Sci. Technol. 2016, 34, 72–82. [Google Scholar] [CrossRef]
- Thielke, M.W.; Secker, C.; Schlaad, H.; Theato, P. Electrospinning of crystallizable polypeptoid fibers. Macromol. Rapid Commun. 2015, 37, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Sener, A.G.; Altay, A.S.; Altay, F. Effect of voltage on morphology of electrospun nanofibers. In Proceedings of the 7th International Conference on Electrical and Electronics Engineering (ELECO), Bursa, Turkey, 1–4 December 2011; pp. I324–I328. [Google Scholar]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y.Ã. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]

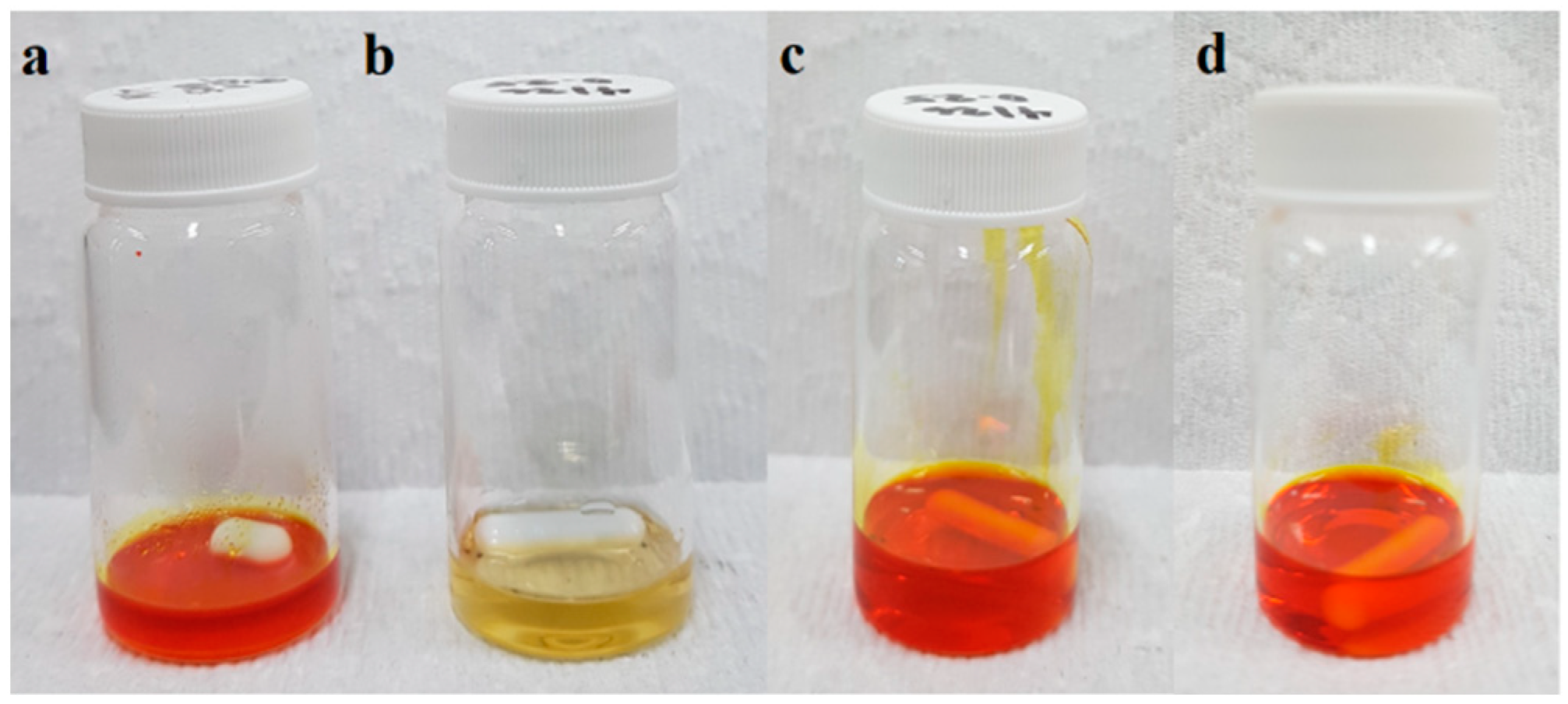







| Polymer Blend | Applied Voltage, kV | |||||
|---|---|---|---|---|---|---|
| 10 | 12 | 14 | 16 | 18 | 20 | |
| SDIB/PBz-0 | ⁄ 1 | |||||
| SDIB/PBz-5 | x 2 | x | x | ⁄ | ||
| SDIB/PBz-10 | x | x | x | x | x | ⁄ |
| Sample | Solvent | Solubility |
|---|---|---|
| ES-SDIB/PBz-0 | DMF, DMSO, DMAc, Ethanol | Insoluble |
| ES-SDIB/PBz-5 | DMF, DMSO, DMAc, Ethanol | Insoluble |
| ES-SDIB/PBz-10 | DMF, DMSO, DMAc, Ethanol | Insoluble |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parreño, R.P., Jr.; Liu, Y.-L.; Beltran, A.B. A Sulfur Copolymers (SDIB)/Polybenzoxazines (PBz) Polymer Blend for Electrospinning of Nanofibers. Nanomaterials 2019, 9, 1526. https://doi.org/10.3390/nano9111526
Parreño RP Jr., Liu Y-L, Beltran AB. A Sulfur Copolymers (SDIB)/Polybenzoxazines (PBz) Polymer Blend for Electrospinning of Nanofibers. Nanomaterials. 2019; 9(11):1526. https://doi.org/10.3390/nano9111526
Chicago/Turabian StyleParreño, Ronaldo P., Jr., Ying-Ling Liu, and Arnel B. Beltran. 2019. "A Sulfur Copolymers (SDIB)/Polybenzoxazines (PBz) Polymer Blend for Electrospinning of Nanofibers" Nanomaterials 9, no. 11: 1526. https://doi.org/10.3390/nano9111526





