CuInS2/Mg(OH)2 Nanosheets for the Enhanced Visible-Light Photocatalytic Degradation of Tetracycline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterization of Catalysts
2.3. Photocatalytic Activity
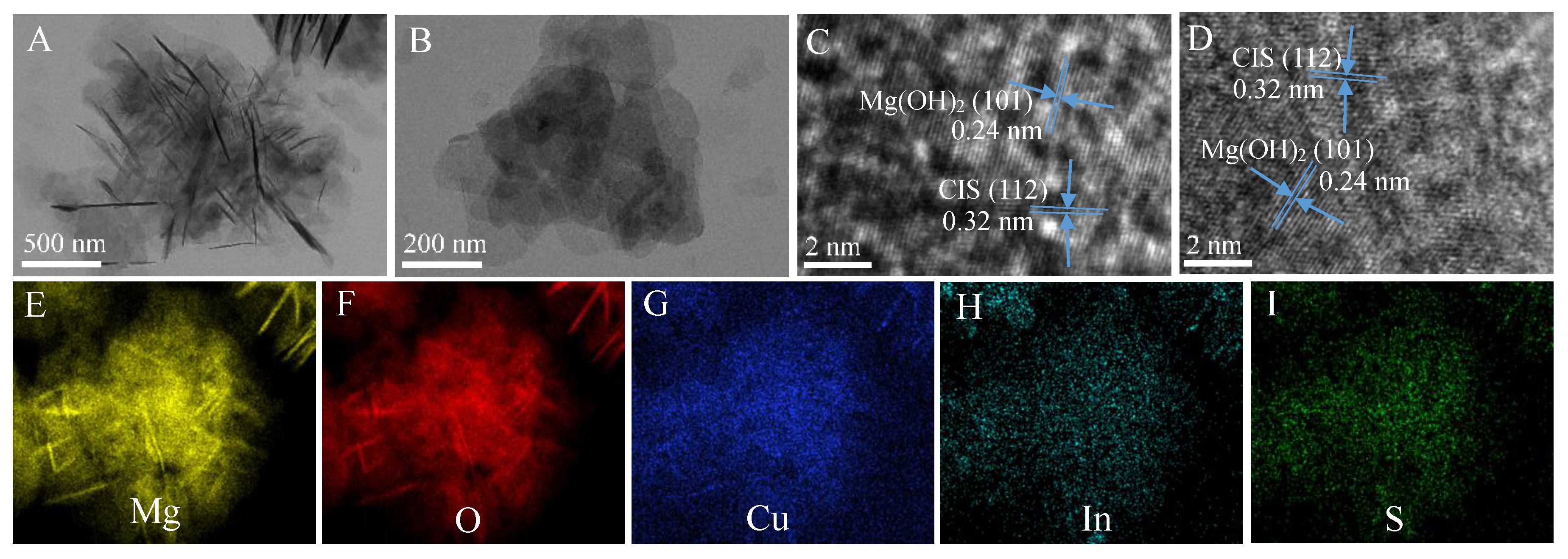
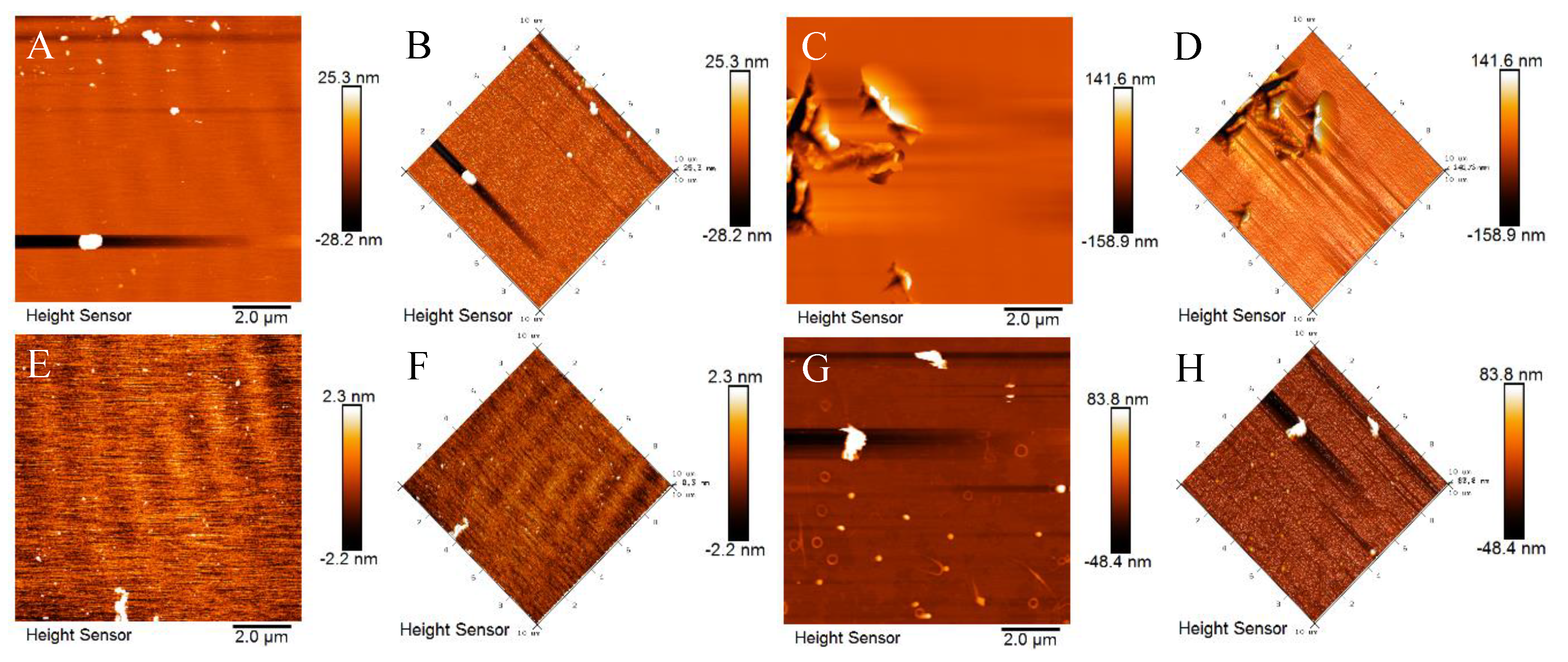
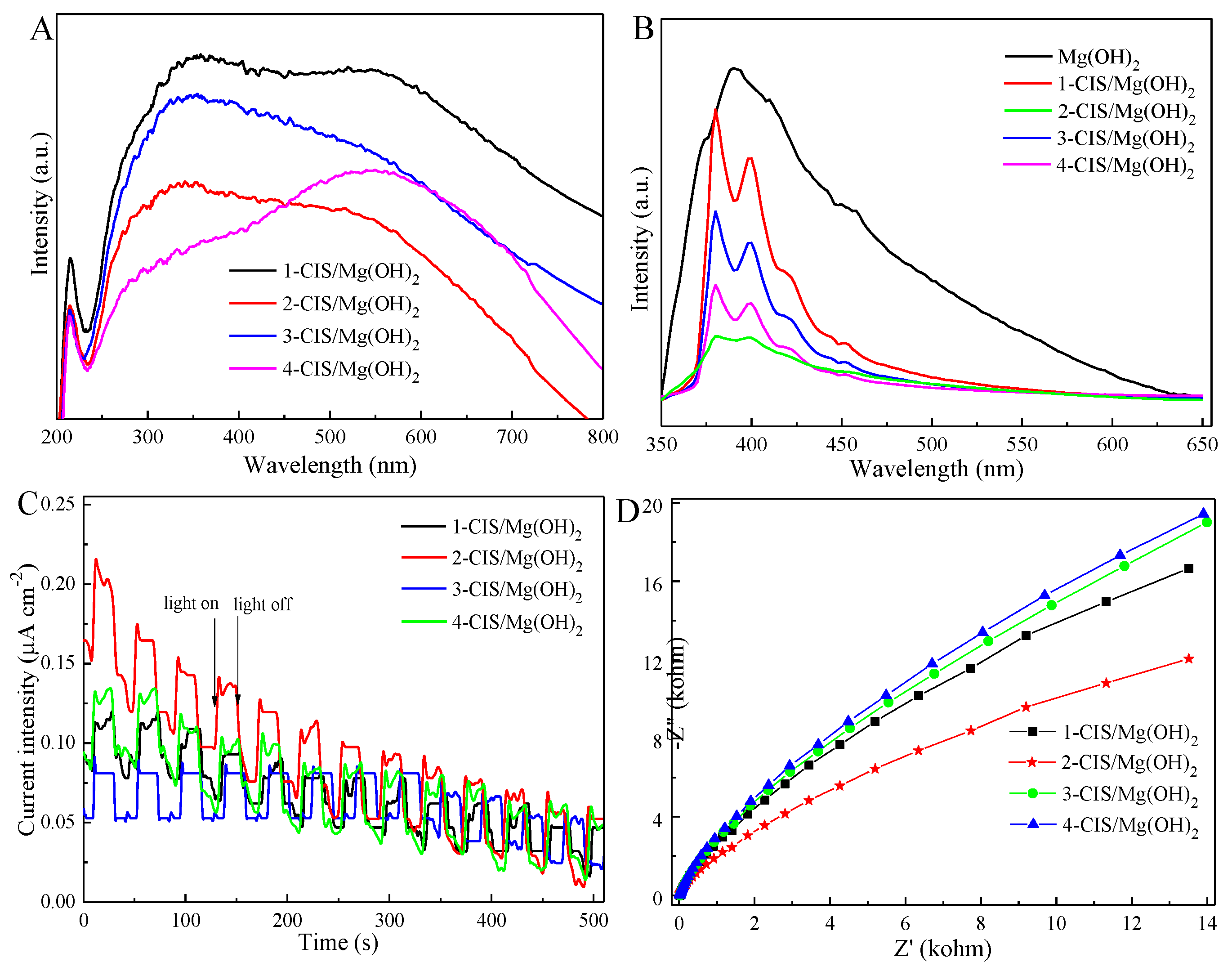
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, J.; Xu, Z.; Chen, Y.; Song, D. Degradation of tetracycline in a schorl/H2O2 system: Proposed mechanism and intermediates. Chemosphere 2018, 202, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Mirsoleimani-Azizi, S.M.; Setoodeh, P.; Zeinali, S.; Rahimpour, M.R. Tetracycline antibiotic removal from aqueous solutions by MOF-5: Adsorption isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2018, 6, 6118–6130. [Google Scholar] [CrossRef]
- Okoli, C.P.; Ofomaja, A.E. Development of sustainable magnetic polyurethane polymer nanocomposite for abatement of tetracycline antibiotics aqueous pollution: Response surface methodology and adsorption dynamics. J. Clean. Prod. 2019, 217, 42–55. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J.; Wu, C.; Zhang, J.; Tang, J.; Shang, J.; Liao, Q. Effect of particle size on adsorption of norfloxacin and tetracycline onto suspended particulate matter in lake. Environ. Pollut. 2019, 244, 549–559. [Google Scholar] [CrossRef]
- Yeşilova, E.; Osman, B.; Kara, A.; Özer, E.T. Molecularly imprinted particle embedded composite cryogel for selective tetracycline adsorption. Sep. Purif. Technol. 2018, 200, 155–163. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, H.; Zhao, Z.; Yu, Y.; Zhou, D.; Dong, S. Model-based evaluation of tetracycline hydrochloride removal and mineralization in an intimately coupled photocatalysis and biodegradation reactor. Chem. Eng. J. 2018, 351, 967–975. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, D.; Zhou, D.; Dong, S.; Wang, J.; Rittman, B.E. Enhancing degradation and mineralization of tetracycline using intimately coupled photocatalysis and biodegradation (ICPB). Chem. Eng. J. 2017, 316, 7–14. [Google Scholar] [CrossRef]
- Saitoh, T.; Shibata, K.; Hiraide, M. Rapid removal and photodegradation of tetracycline in water by surfactant-assisted coagulation—Sedimentation method. J. Environ. Chem. Eng. 2014, 2, 1852–1858. [Google Scholar] [CrossRef]
- Saitoh, T.; Shibata, K.; Fujimori, K.; Ohtani, Y. Rapid removal of tetracycline antibiotics from water by coagulation-flotation of sodium dodecyl sulfate and poly (allylamine hydrochloride) in the presence of Al(III) ions. Sep. Purif. Technol. 2017, 187, 76–83. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Fang, Z.; Tsang, E.P. Ceria accelerated nanoscale zerovalent iron assisted heterogenous Fenton oxidation of tetracycline. Chem. Eng. J. 2019, 369, 588–599. [Google Scholar] [CrossRef]
- Taşkan, B.; Casey, E.; Hasar, H. Simultaneous oxidation of ammonium and tetracycline in a membrane aerated biofilm reactor. Sci. Total Environ. 2019, 682, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway. Chem. Eng. J. 2019, 369, 745–757. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Shah, K.J.; Chiang, P.C.; Zheng, H. Electrocatalytic oxidation of tetracycline by Bi-Sn-Sb/γ-Al2O3 three-dimensional particle electrode. J. Hazard. Mater. 2019, 370, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, K.; Huang, Z.; Liu, Y.; Wen, J.; Peng, H. MgO nanosheets with N-doped carbon coating for the efficient visible-light photocatalysis. J. Ind. Eng. Chem. 2019, 76, 288–295. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, W.; Kang, F.; Peng, H.; Wen, J. Enhanced photo-Fenton degradation of tetracycline using TiO2-coated α-Fe2O3 core-shell heterojunction. J. Ind. Eng. Chem. 2018, 68, 14–23. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, C.; Hou, J.; Yuan, S.; Wang, Y.; Xu, P.; Gao, G.; Shi, J. 3D hollow hierarchical structures based on 1D BiOCl nanorods intersected with 2D Bi2WO6 nanosheets for efficient photocatalysis under visible light. Nanomaterials 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Rui, Y.; Sun, K.; Cui, W.; An, W. Surface Decoration of ZnWO4 nanorods with Cu2O nanoparticles to build heterostructure with enhanced photocatalysis. Nanomaterials 2018, 8, 33. [Google Scholar] [CrossRef]
- Ye, G.; Yu, Z.; Li, Y.; Li, L.; Song, L.; Gu, L.; Cao, X. Efficient treatment of brine wastewater through a flow-through technology integrating desalination and photocatalysis. Water Res. 2019, 157, 134–144. [Google Scholar] [CrossRef]
- Negrin-Montecelo, Y.; Testa-Anta, M.; Marin-Caba, L.; Perez-Lorenzo, M.; Salgueirino, V.; Correa-Duarte, M.A.; Comesana-Hermo, M. Titanate nanowires as one-dimensional hot spot generators for broadband Au-TiO2 photocatalysis. Nanomaterials 2019, 9, 990. [Google Scholar] [CrossRef]
- Hao, M.; Deng, X.; Xu, L.; Li, Z. Noble metal Free MoS2/ZnIn2S4 nanocomposite for acceptorless photocatalytic semi-dehydrogenation of 1,2,3,4-tetrahydroisoquinoline to produce 3,4-dihydroisoquin-oline. Appl. Catal. B Environ. 2019, 252, 18–23. [Google Scholar] [CrossRef]
- Yan, L.; Li, Z.; Sun, M.; Shen, G.; Li, L. Stable and flexible CuInS2/ZnS:Al-TiO2 film for solar-light-driven photodegradation of soil fumigant. ACS Appl. Mater. Interf. 2016, 8, 20048–20056. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Deng, F.; Liu, M.; Luo, X.; Wang, A. The regulation on visible-light photocatalytic activity of CuInS2 by different Cu/In molar ratio. Mater. Chem. Phys. 2018, 212, 372–377. [Google Scholar] [CrossRef]
- Leach, A.D.P.; Mast, L.G.; Hernández-Pagán, E.A.; Macdonald, J.E. Phase dependent visible to near-infrared photoluminescence of CuInS2 nanocrystals. J. Mater. Chem. C 2015, 3, 3258–3265. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhan, X.; Wang, F.; Wang, Q.; Xu, K.; Liu, Q.; Jiang, C.; Wang, Z.; He, J. Construction of CuInS2/Ag sensitized ZnO nanowire arrays for efficient hydrogen generation. RSC Adv. 2015, 5, 81723–81727. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Nie, Q.; Liu, B.; Liu, E.; Hu, X.; Fan, J. Aqueous-synthesis of CuInS2 core and CuInS2/ZnS core/shell quantum dots and their optical properties. Mater. Lett. 2017, 200, 27–30. [Google Scholar] [CrossRef]
- Enesca, A.; Yamaguchi, Y.; Terashima, C.; Fujishima, A.; Nakata, K.; Duta, A. Enhanced UV-Vis photocatalytic performance of the CuInS2/TiO2/SnO2 hetero-structure for air decontamination. J. Catal. 2017, 350, 174–181. [Google Scholar] [CrossRef]
- Liu, A.; Yu, C.; Lin, J.; Sun, G.; Xu, G.; Huang, Y.; Liu, Z.; Tang, C. Construction of CuInS2@ZIF-8 nanocomposites with enhanced photocatalytic activity and durability. Mater. Res. Bull. 2019, 112, 147–153. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.M.; Salavati-Niasari, M.; Mohandes, F. CuInS2 nanostructures: Synthesis, characterization, formation mechanism and solar cell applications. J. Ind. Eng. Chem. 2014, 20, 3800–3807. [Google Scholar] [CrossRef]
- Kong, L.; Li, Z.; Huang, S.; Jia, J.; Li, L. Boosting photocatalytic performance and stability of CuInS2/ZnS-TiO2 heterostructures via sol-gel processed integrate amorphous titania gel. Appl. Catal. B Environ. 2017, 204, 403–410. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Li, M.; Shi, Y.; Wen, H. 2D/2D Z-scheme heterojunction of CuInS2/g-C3N4 for enhanced visiblelight-driven photocatalytic activity towards the degradation of tetracycline. Sep. Purif. Technol. 2019, 210, 608–615. [Google Scholar] [CrossRef]
- Luo, S.; Ke, J.; Yuan, M.; Zhang, Q.; Xie, P.; Deng, L.; Wang, S. CuInS2 quantum dots embedded in Bi2WO6 nanoflowers for enhanced visible light photocatalytic removal of contaminants. Appl. Catal. B Environ. 2018, 221, 215–222. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Deng, F.; Luo, X.; Gao, J.; Dionysiou, D.D. Unique surface structure of nano-sized CuInS2 anchored on rGO thin film and its superior photocatalytic activity in real wastewater treatment. Chem. Eng. J. 2018, 338, 591–598. [Google Scholar] [CrossRef]
- Li, J.; Kempken, B.; Dzhagan, V.; Zahn, D.R.T.; Grzelak, J.; Mackowski, S.; Parisia, J.; Kolny-Olesiak, J. Alloyed CuInS2–ZnS nanorods: Synthesis, structure and optical properties. CrystEngComm 2015, 17, 5634–5643. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Cai, X.; Yu, W.; Zhang, L.; Zhao, R.; Ke, S. Blue phosphorus/Mg(OH)2 van der Waals heterostructures as promising visible-light photocatalysts for water splitting. J. Phys. Chem. C 2018, 122, 7075–7080. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres Martínez, L.M. Mg(OH)2 films prepared by ink-jet printing and their photocatalytic activity in CO2 reduction and H2O conversion. Top. Catal. 2018, 61, 1574–1584. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Ren, K.; Yu, J.; Tang, W. A two-dimensional vertical van der Waals heterostructure based on g-GaN and Mg(OH)2 used as a promising photocatalyst for water splitting: A first-principles calculation. J. Appl. Phys. 2019, 126, 065701. [Google Scholar] [CrossRef]
- Flores-Flores, M.; Luévano-Hipólito, E.; Torres-Martíneza, L.M.; Do, T.O. CO2 adsorption and photocatalytic reduction over Mg(OH)2/CuO/Cu2O under UV-Visible light to solar fuels. Mater. Chem. Phys. 2019, 227, 90–97. [Google Scholar] [CrossRef]
- Yagmurcukardes, M.; Torun, E.; Senger, R.T.; Peeters, F.M.; Sahin, H. Mg(OH)2-WS2 van der Waals heterobilayer: Electric field tunable band-gap crossover. Phys. Rev. B 2016, 94, 195403. [Google Scholar] [CrossRef]
- Wang, F.; Cui, A.; Sun, H.; Zhou, B.; Xu, L.; Jiang, K.; Shang, L.; Hu, Z.; Chu, J. Electronic bandgap manipulation of monolayer WS2 by vertically coupled insulated Mg(OH)2 layers. J. Alloy. Compd. 2019, 785, 156–162. [Google Scholar] [CrossRef]
- Suslu, A.; Wu, K.; Sahin, H.; Chen, B.; Yang, S.; Cai, H.; Aoki, T.; Horzum, S.; Kang, J.; Peeters, F.M. Unusual dimensionality effects and surface charge density in 2D Mg(OH)2. Sci. Rep. 2016, 6, 20525. [Google Scholar] [CrossRef] [PubMed]
- Bacaksiz, C.; Dominguez, A.; Rubio, A.; Senger, R.T.; Sahin, H. h-AlN-Mg(OH)2 van der Waals bilayer heterostructure: Tuning the excitonic characteristics. Phys. Rev. B 2017, 95, 075423. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; You, Y.; Fu, X.; Liu, Y.; Wen, J. One-pot synthesis of sandwich-like MgO@Carbon with enhanced sorption capacity of organic dye. Chem. Eng. J. 2018, 334, 1399–1409. [Google Scholar] [CrossRef]
- Ren, K.; Yu, J.; Tang, W. First-principles study of two-dimensional van der Waals heterostructure based on ZnO and Mg(OH)2: A potential photocatalyst for water splitting. Phys. Lett. A 2019, 383, 125916. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.; Ren, K.; Chou, J.; Yu, J.; Sun, Z.; Sun, M. Transition-metal dichalcogenides/Mg(OH)2 van der Waals heterostructures as promising water-splitting photocatalysts: A first-principles study. Phys. Chem. Chem. Phys. 2019, 21, 1791. [Google Scholar] [CrossRef]
- Xia, C.; Xiong, W.; Du, J.; Wang, T.; Wei, Z.; Li, J. Robust electronic and mechanical properties to layer number in 2D wide-gap X(OH)2 (X=Mg, Ca). J. Phys. Appl. Phys. 2018, 51, 015107. [Google Scholar] [CrossRef]
- Wu, B.; Lu, S.; Xu, W.; Cui, S.; Li, J.; Han, P. Study on corrosion resistance and photocatalysis of cobalt superhydrophobic coating on aluminum substrate. Surf. Coat. Technol. 2017, 350, 174–181. [Google Scholar] [CrossRef]
- Liu, X.; Lv, S.; Fan, B.; Xing, A.; Jia, B. Ferroelectric polarization-enhanced photocatalysis in BaTiO3-TiO2 core-shell heterostructures. Nanomaterials 2019, 9, 1116. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Huang, Z.; Zheng, X.; Wen, J. Enhanced photo-Fenton activity of Sm2O3–NiO heterojunction under visible light irradiation. J. Alloy. Compd. 2019, 800, 498–504. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Peng, H.; Wen, J. Ag-decorated core-shell Sm2O3@TiO2 nanocomposites with enhanced visible-light photocatalytic performance. J. Phys. Chem. Solids 2018, 123, 206–215. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Z.; Peng, H.; Zheng, X. Core-shell Sm2O3@ZnO nano-heterostructure for the visible light driven photocatalytic performance. Coll. Surf. A 2019, 560, 244–251. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; You, Y.; Peng, H.; Wen, J. Core-shell structured α-Fe2O3@CeO2 heterojunction for the enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2018, 101, 20–28. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Effect of inorganic ions on the ultrasound initiated degradation and product formation of triphenylmethane dyes. Ultrason. Sonochem. 2018, 48, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, J.; Wang, Y. Combination of photocatalysis with hydrodynamic cavitation for degradation of tetracycline. Chem. Eng. J. 2017, 315, 274–282. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Liu, P.; Yan, J.H.; Yang, G.W. Matching energy levels between TiO2 and α-Fe2O3 in a core-shell nanoparticle for the visible-light photocatalysis. J. Mater. Chem. A 2015, 3, 14853–14863. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.M.; Al-Shirbini, A.S.; Mohamed, O.; Nasr, O. Photocatalytic degradation of paracetamol over magnetic flower-like TiO2/Fe2O3 core-shell nanostructures. J. Photochem. Photobiol. A 2017, 347, 186–198. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D flowerlike α-Fe2O3@TiO2 core-shell nanostructures: General synthesis and enhanced photocatalytic performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Chen, M.; Shen, X.; Wu, Q.H.; Li, W.; Diao, G.W. Template-assisted synthesis of core-shell α-Fe2O3@TiO2 nanorods and their photocatalytic property. J. Mater. Sci. 2015, 50, 4083–4094. [Google Scholar] [CrossRef]
- Reyes, C.; Fernández, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A 2006, 184, 141–146. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Mao, Y.; Wen, J.; Fu, X.; Liu, X. CuInS2/Mg(OH)2 Nanosheets for the Enhanced Visible-Light Photocatalytic Degradation of Tetracycline. Nanomaterials 2019, 9, 1567. https://doi.org/10.3390/nano9111567
Zheng X, Mao Y, Wen J, Fu X, Liu X. CuInS2/Mg(OH)2 Nanosheets for the Enhanced Visible-Light Photocatalytic Degradation of Tetracycline. Nanomaterials. 2019; 9(11):1567. https://doi.org/10.3390/nano9111567
Chicago/Turabian StyleZheng, Xiaogang, Yiting Mao, Jing Wen, Xiaojin Fu, and Xinhui Liu. 2019. "CuInS2/Mg(OH)2 Nanosheets for the Enhanced Visible-Light Photocatalytic Degradation of Tetracycline" Nanomaterials 9, no. 11: 1567. https://doi.org/10.3390/nano9111567
APA StyleZheng, X., Mao, Y., Wen, J., Fu, X., & Liu, X. (2019). CuInS2/Mg(OH)2 Nanosheets for the Enhanced Visible-Light Photocatalytic Degradation of Tetracycline. Nanomaterials, 9(11), 1567. https://doi.org/10.3390/nano9111567





