Recent Advances and Future Prospects in Spark Plasma Sintered Alumina Hybrid Nanocomposites
Abstract
1. Introduction
2. Synthesis of Alumina Hybrid Nanocomposite Powders
2.1. Wet Dispersion and Sonication
2.2. Ball Milling
2.3. Molecular Level Mixing
2.4. Sole-Gel Method
2.5. Colloidal Processing
3. Consolidation of Alumina Hybrid Nanocomposite Powders
4. Spark Plasma Sintering Method
4.1. The SPS Process
4.2. Densification
5. Mechanical Properties
5.1. Hardness and Strength
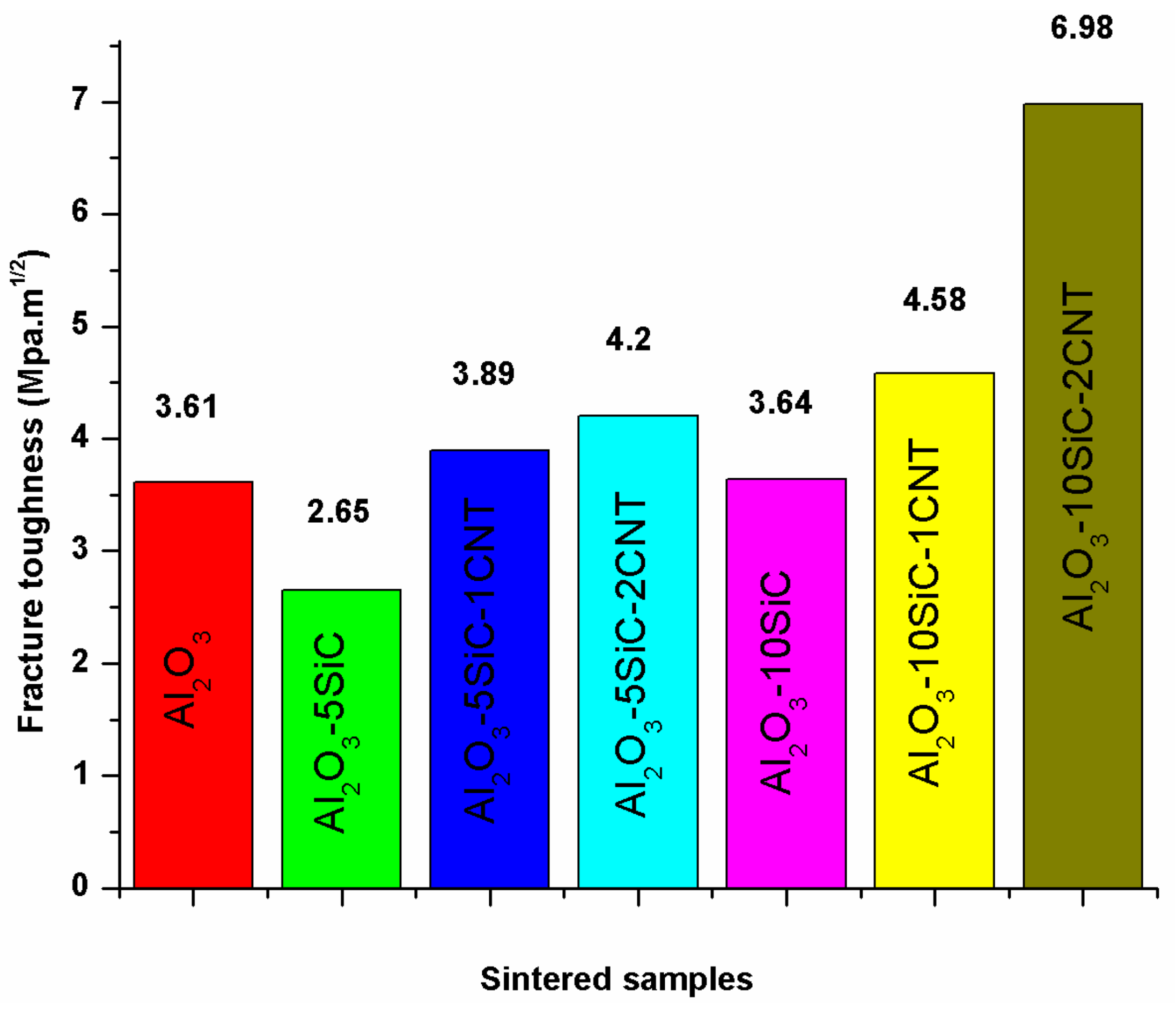
5.2. Fracture Toughness
5.3. Wear and Friction
6. Physical Properties
6.1. Electrical Conductivity
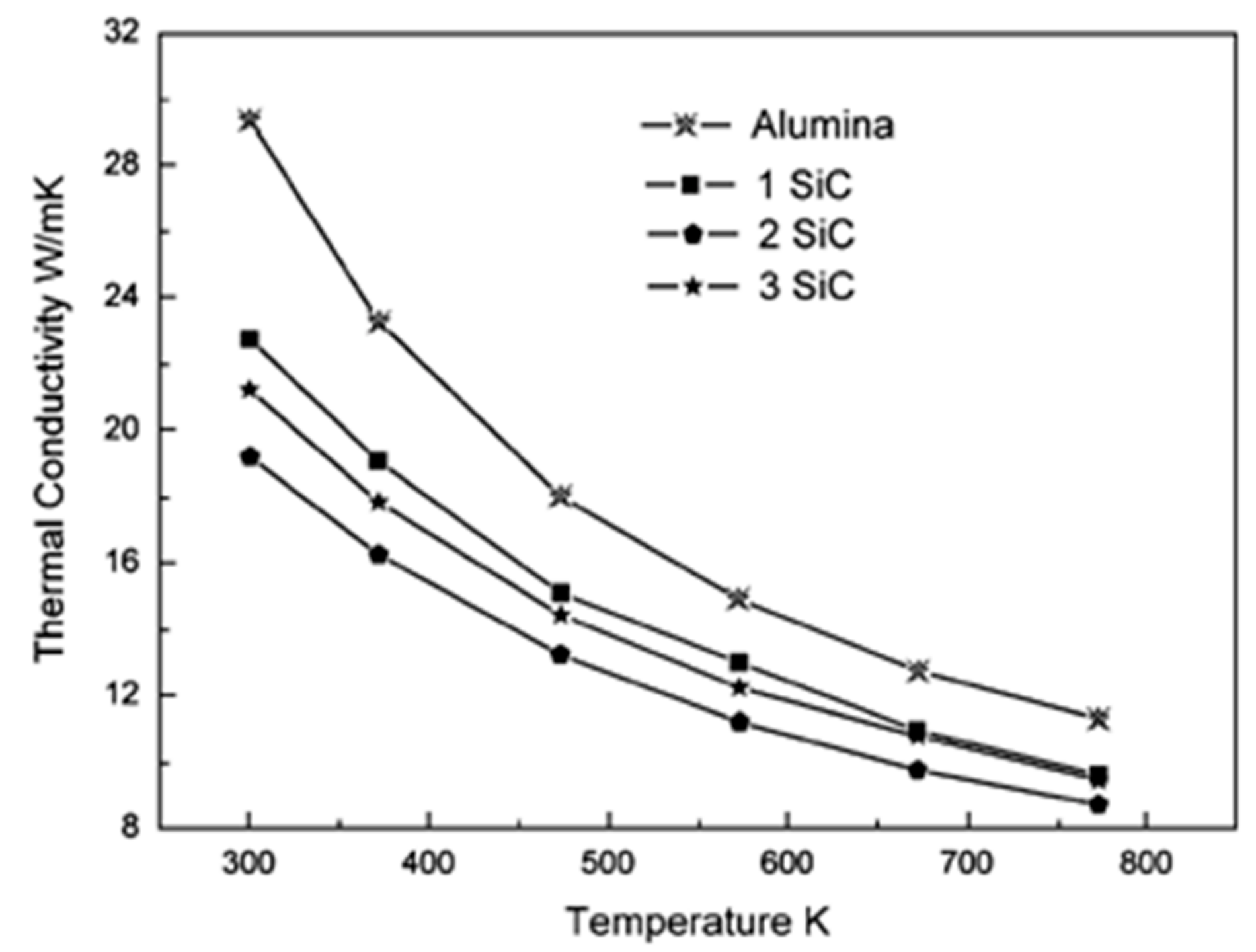
6.2. Thermal Conductivity
7. Spark Plasma Sintered Alumina
8. Spark Plasma Sintered Alumina Hybrid Nanocomposites
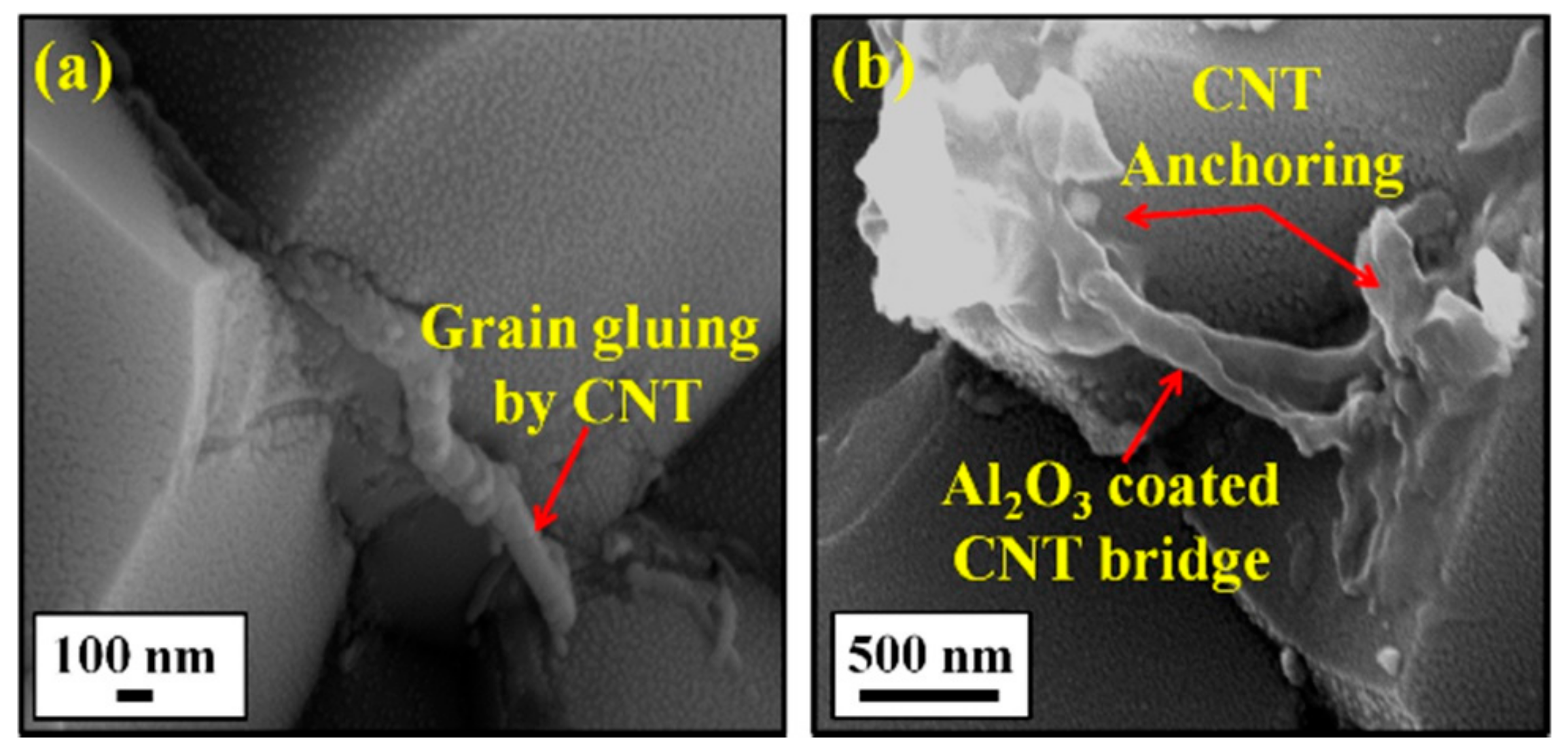
8.1. Al2O3-SiC-CNTs
8.2. Alumina-SiC-Graphene
8.3. Alumina-Graphene-CNTs
8.4. Alumina-Modified Multilayer Graphene Nanoplatelets
8.5. Al2O3–ZrO2-Graphene
8.6. Al2O3-SiCw-TiC
8.7. Al2O3-TiC-Ni
8.8. Al2O3-CNFs-SiC
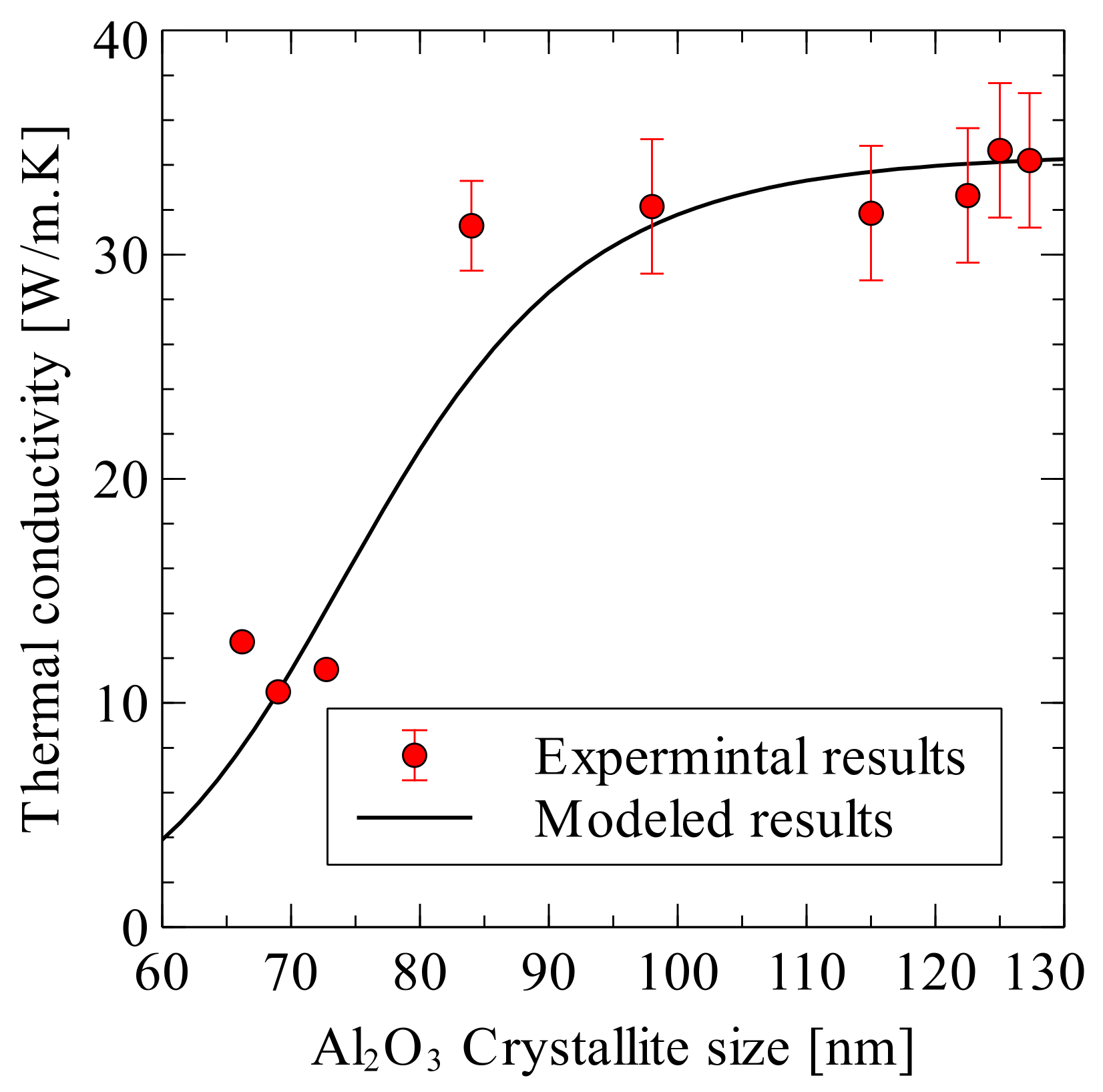
8.9. Modelling of Spark Plasma Sintered Alumina Hybrid Nanocomposites
8.10. Overall Change in Properties
9. Potential Applications
10. Future Directions
- (a)
- (b)
- The possibility of introducing innovative nanocomposite powder synthesis techniques needs to be further explored. For instance, the MLM process could be easily used to prepare new hybrid ceramic nanocomposites, including alumina reinforced by carbonaceous materials, such as carbon nanotubes and graphene.
- (c)
- The uniform distribution of the nanoreinforcements in the matrix should be ascertained with the use of advanced and complementary characterization techniques, such as FE-SEM and TEM.
- (d)
- SPS process parameters need to be optimized to prepare fully dense materials that have preserved nanostructures and are free from pores and flaws.
- (e)
- A fundamental understanding of sintering mechanisms is necessary, particularly when the insulating alumina matrix is strengthened by nanoreinforcements that have extremely high electrical and thermal conductivities.
- (f)
- Proper engineering of the interface between the reinforcement and matrix, through careful design of synthesis and processing procedures, is a pre-requisite to have materials with the anticipated properties. This is because the interface influences the load, electron, and phonon transfer among the constituents of the hybrid composite. The quality of the interface needs to be assessed and the interfacial shear stress needs to be measured to have better understating of their influence on the composites’ bulk properties.
- (g)
- Intrinsic and extrinsic strengthening, toughening, and transport mechanisms operating at different length scales and responsible for the change in the properties need comprehensive discussion supported by experimental evidence.
- (h)
- Despite the practical importance of the thermal, electrical, and tribological properties in many applications of alumina hybrid nanocomposites, only limited experimental data are available to date. Therefore, these properties need to be systematically measured and thoroughly understood.
- (i)
- So far, the effect of oxygen stoichiometry on the mechanical and electronic properties has not been investigated. Research work, which would consider this point, is critically needed.
- (j)
- For submicron and nanoscale crystallites, the electrical properties of compounds significantly vary with the same chemical composition; therefore, the effect of the size of real crystallites on the electronic properties of hybrid nanocomposites needs to be investigated.
- (k)
- The fracture toughness of the hybrid nanocomposite materials should be measured while using standard testing methods, such as the single-edged-notched-beam, to obtain reliable data and reduce the discrepancies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelleg, J. Mechanical Properties of Ceramics; Springer International Publishing Switzerland: Cham, Switzerland, 2014. [Google Scholar]
- Kelly, A. Composites in context. Compos. Sci. Technol. 1985, 23, 171–199. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chunyu, L.; Chou, T.W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Ashby, M.F.; Ferreira, P.J.; Schodek, D.L. Nanomaterials, Nanotechnologies and Design: An Introduction for Engineers and Architects; Elsevier Ltd.: Rio de Janeiro, RJ, USA, 2009. [Google Scholar]
- Niihara, K. New design concept of structural ceramics/ceramic nanocomposites. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Palmero, P. Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods. Nanomaterials 2015, 5, 656–696. [Google Scholar] [CrossRef] [PubMed]
- Munro, M. Evaluated Material Properties for a Sintered α-Alumina. J. Am. Ceram. Soc. 1997, 80, 1919–1928. [Google Scholar] [CrossRef]
- Liu, B.Q.; Huang, C.Z.; Sun, A.L. Toughening Mechanisms and Wear Behavior of a TiC Whisker Toughening Alumina Ceramic Cutting Tool Composite. Adv. Mater. Res. 2012, 500, 634–639. [Google Scholar] [CrossRef]
- Mozalev, A.; Sakairi, M.; Takahashi, H.; Habazaki, H.; Hubálek, J. Nanostructured anodic-alumina-based dielectrics for high-frequency integral capacitors. J. Thin. Solid Film. 2014, 550, 486–494. [Google Scholar] [CrossRef]
- Martin, C.A.; Lee, G.F.; Fedderly, J.J. Composite armor system including a ceramic-embedded heterogeneously layered polymeric matrix. US Patent 8, 387, 510, 5 March 2013. [Google Scholar]
- Ahmad, I.; Yazdani, B.; Zhu, Y. Recent Advances on Carbon Nanotubes and Graphene Reinforced Ceramics Nanocomposites. Nanomaterials 2015, 5, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Niihara, K. Microstructure and properties of alumina-silicon carbide nanocomposites fabricated by pressureless sintering and post hot-isostatic pressing. Trans. Nonferrous Metals Soc. China 2011, 21, s1–s6. [Google Scholar] [CrossRef]
- Ohji, T.; Nakahira, A.; Hirano, T.; Niihara, K. Tensile creep behaviour of alumina/silicon carbide nanocomposite. J. Am. Ceram. Soc. 1994, 77, 3259–3262. [Google Scholar] [CrossRef]
- Ohji, T.; Hirano, T.; Nankahira, A.; Niihara, K. Particle/matrix interface and its role in creep inhibition in alumina/silicon carbide nanocomposites. J. Am. Ceram. Soc. 1996, 79, 33–45. [Google Scholar] [CrossRef]
- Ohji, T.; Jeong, J.K.; Choa, Y.H.; Niihar, K. Strengthening and toughening mechanisms of ceramic nanocomposites. J. Am. Ceram. Soc. 1998, 81, 1453–1460. [Google Scholar] [CrossRef]
- Derby, B. Ceramic nanocomposites: Mechanical properties. Curr. Opin. Solid State Merter. 1998, 3, 490–495. [Google Scholar] [CrossRef]
- Sternitzke, M. Review: Structural ceramic nanocomposites. J. Eur. Ceram. Soc. 1997, 17, 1061–1082. [Google Scholar] [CrossRef]
- Sommer, F.; Landfried, R.; Kern, F.; Gadow, R. Mechanical properties of zirconia toughened alumina with 10-24 vol.% 1Y-TZP reinforcement. J. Eur. Ceram. Soc. 2012, 32, 4177–4184. [Google Scholar] [CrossRef]
- Su, X.; Zhou, J.; Wang, B.; Zhao, P. Synthesis and electric field-assisted sintering behavior of Al2O3-ZrO2 composite nanopowders by polyacrylamide gel method. J. Sol-Gel Sci. Technol. 2016, 80, 126–132. [Google Scholar] [CrossRef]
- Fan, K.; Pastor, J.Y.; Ruiz-Hervias, J.; Gurauskis, J.; Baudin, C. Determination of mechanical properties of Al2O3/Y-TZP ceramic composites: Influence of testing method and residual stresses. Ceram. Int. 2016, 42, 18700–18710. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Lee, K.H.; Mo, C.B.; Hong, S.H. Strengthening and toughening of carbon nanotube reinforced alumina nanocomposite fabricated by molecular level mixing process. Scr. Mater. 2005, 53, 793–797. [Google Scholar] [CrossRef]
- Wang, W.; Yamamoto, G.; Shirasu, K.; Nozaka, Y.; Hashida, T. Effects of processing conditions on microstructure, electrical conductivity and mechanical properties of MWCNT/alumina composites prepared by flocculation. J. Eur. Ceram. Soc. 2015, 35, 3903–3908. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Li, W. Colloidal Processing of Carbon Nanotube/Alumina Composites. Chem. Mater. 2002, 14, 5169–5172. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.C.; Li, J.L.; Wang, L.J.; Jiang, W. Preparation and Mechanical Properties of Graphene Nanosheet Reinforced Alumina Composites. Adv. Eng. Mater. 2015, 17, 28–35. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, C.F.; Smirnov, A.; Centeno, A.; Fernández, A.; Alonso, B.; Rocha, V.G.; Torrecillas, R.; Zurutuza, A.; Bartolome, J.F. Wear behavior of graphene/alumina composite. Ceram. Int. 2015, 41, 7434–7438. [Google Scholar] [CrossRef]
- Lee, B.; Koo, M.Y.; Jin, S.H.; Kim, K.T.; Hong, S.H. Simultaneous strengthening and toughening of reduced graphene oxide/alumina composites fabricated by molecular-level mixing process. Carbon 2014, 78, 212–219. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Hassanin, H.; Jumbu, N.; Deng, S.; Zuo, Q.; Jiang, K. Graphene–Alumina Nanocomposites with Improved Mechanical Properties for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Pan, W.; Wu, H. High performance alumina based graphene nanocomposites with novel electrical and dielectric properties. RSC Adv. 2015, 5, 33607–33614. [Google Scholar] [CrossRef]
- Palmero, P.; Kern, F.; Sommer, F.; Lombardi, M.; Gadow, R.; Montanaro, L. Issues in nanocomposite ceramic engineering: Focus on processing and properties of alumina-based composites. J. Appl. Biomater. Funct. Mater. 2014, 12, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Galusek, D.; Galusková, D. Alumina Matrix Composites with Non-Oxide Nanoparticle Addition and Enhanced Functionalities. Nanomaterials 2015, 5, 115–143. [Google Scholar] [CrossRef] [PubMed]
- Estili, M.; Sakka, Y. Recent advances in understanding the reinforcing ability and mechanism of carbon nanotubes in ceramic matrix composites. Sci. Technol. Adv. Mater. 2014, 15, 064902. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Pan, W. Hybrid nanocomposites: A new route towards tougher alumina ceramics. Compos. Sci. Technol. 2008, 68, 1321–1327. [Google Scholar] [CrossRef]
- Saheb, N.; Qadir, N.U.; Siddiqui, M.U.; Arif, A.F.M.; Akhtar, S.S.; Al-Aqeeli, N. Characterization of nanoreinforcement dispersion in inorganic nanocomposites: A review. Materials 2014, 7, 4148–4181. [Google Scholar] [CrossRef] [PubMed]
- Sciti, D.; Vicens, J.; Bellosi, A. Microstructure and properties of alumina-SiC nanocomposites prepared from ultrafine powders. J. Mater. Sci. 2002, 37, 3747–3758. [Google Scholar] [CrossRef]
- Yazdani, B.; Xia, Y.; Ahmad, I.; Zhu, Y. Graphene and carbon nanotube (GNT)-reinforced alumina nanocomposites. J. Eur. Ceram. Soc. 2015, 35, 179–186. [Google Scholar] [CrossRef]
- Aguilar-Elguézabal, A.; Bocanegra-Bernal, M.H. Fracture behaviour of α-Al2O3 ceramics reinforced with a mixture of single-wall and multi-wall carbon nanotubes. Compos. Part B 2014, 60, 463–470. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Aqeeli, N. Mechanically alloyed nanocomposites. Prog. Mater. Sci. 2013, 58, 383–564. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yoon, D.H. Properties of alumina matrix composites reinforced with SiC whisker and carbon nanotubes. Ceram. Int. 2014, 40, 14375–14383. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Yan, H.; Jiang, K. Spark Plasma Sintering of Alumina Composites with Graphene Platelets and Silicon Carbide Nanoparticles. Adv. Eng. Mater. 2014, 16, 1111–1118. [Google Scholar] [CrossRef]
- Ahmad, K.; Pan, W.; Qu, Z. Multifunctional Properties of Alumina Composites Reinforced by a Hybrid Filler. Int. J. Appl. Ceram. Technol. 2009, 6, 80–88. [Google Scholar] [CrossRef]
- Saheb, N.; Mohammad, K. Microstructure and mechanical properties of spark plasma sintered Al2O3-SiC-CNTs hybrid nanocomposites. Ceram. Int. 2016, 42, 12330–12340. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Arshad, S.N.; Mo, C.B.; Hong, S.H. Extraordinary Strengthening Effect of Carbon Nanotubes in Metal-Matrix Nanocomposites Processed by Molecular-Level Mixing. Adv. Mater. 2005, 17, 1377–1381. [Google Scholar] [CrossRef]
- Mohammad, K.; Saheb, N. Molecular level mixing: An approach for synthesis of homogenous hybrid ceramic nanocomposite powders. Powder Technol. 2016, 291, 121–130. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Gómez-García, D.; Domínguez-Rodríguez, A. Towards physical properties tailoring of carbon nanotubes-reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2012, 32, 3001–3020. [Google Scholar] [CrossRef]
- Mo, C.B.; Cha, S.I.; Kim, K.T.; Lee, K.H.; Hong, S.H. Fabrication of carbon nanotube reinforced alumina matrix nanocomposite by sol-gel process. Mater. Sci. Eng. A 2005, 395, 124–128. [Google Scholar] [CrossRef]
- Mohanty, P.; Mohapatra, S.; Mohapatra, J.; Singh, S.K.; Padhi, P.; Mishra, D.K. Utilization of Chemically Synthesized Fine Powders of SiC/Al2O3 Composites for Sintering. Mater. Manuf. Process. 2016, 29, 1–7. [Google Scholar]
- Crisan, M.; Jitianu, A.; Zaharescu, M.; Mizukami, F.; Niwa, S. Sol-Gel Mono- and Poly-component Nanosized Powders in the Al2O3-TiO2-SiO2-MgO System. J. Dispers. Sci. Technol. 2003, 24, 129–144. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Subhani, T.; Saeed, K.; Islam, M.; Wang, N.; Zhu, Y. Synergic influence of MWCNTs and SiC nanoparticles on the microstructure and properties of Al2O3 ceramic hybrid nanocomposites. Curr. Appl. Phys. 2016, 16, 1649–1658. [Google Scholar] [CrossRef]
- Ahmad, I.; Cao, H.; Chen, H.; Zhao, H.; Kennedy, A.; Zhu, Y.Q. Carbon nanotube toughened aluminium oxide nanocomposite. J. Eur. Ceram. Soc. 2010, 30, 865–873. [Google Scholar] [CrossRef]
- Kanamaru, M.; Tatsuno, T.; Kusaka, T. Hot-Pressed Al2O3/SiC Whisker/TiC Nano-Composites. J. Ceram. Soc. Jpn. 1992, 100, 408–412. [Google Scholar] [CrossRef][Green Version]
- Yazdani, B.; Xu, F.; Ahmad, I.; Hou, X.; Xia, Y.; Zhu, Y. Tribological performance of Graphene/Carbon nanotube hybrid reinforced Al2O3 composites. Sci. Rep. 2015, 5, 11579. [Google Scholar] [CrossRef] [PubMed]
- Michálek, M.; Sedláček, J.; Parchovianský, M.; Michálková, M.; Galusek, D. Mechanical properties and electrical conductivity of alumina/MWCNT and alumina/zirconia/MWCNT composites. Ceram. Int. 2014, 40, 1289–1295. [Google Scholar] [CrossRef]
- Yazdani, B.; Porwal, H.; Xia, Y.; Yan, H.; Reece, M.J.; Zhu, Y. Role of synthesis method on microstructure and mechanical properties of graphene/carbon nanotube toughened Al2O3 nanocomposites. Ceram. Int. 2015, 41, 9813–9822. [Google Scholar] [CrossRef]
- Gutiérrez-González, C.F.; Suarez, M.; Pozhidaev, S.; Rivera, S.; Peretyagin, P.; Solís, W.; Díaz, L.A.; Fernandez, A.; Torrecillas, R. Effect of TiC addition on the mechanical behaviour of Al2O3–SiC whiskers composites obtained by SPS. J. Eur. Ceram. Soc. 2016, 36, 2149–2152. [Google Scholar] [CrossRef]
- Pozhidaev, S.S.; Seleznev, A.E.; Pinargote, N.W.S.; Peretyagin, P.Y. Spark plasma sintering of electro conductive nanocomposite Al2O3-SiCw-TiC. Mech. Ind. 2015, 16, 1–6. [Google Scholar] [CrossRef]
- Rodriguez-Suarez, T.; Bartolomé, J.F.; Smirnov, A.; Lopez-Esteban, S.; Díaza, L.A.; Torrecillas, R.; Moya, J.S. Electroconductive Alumina–TiC–Ni nanocomposites obtained by Spark Plasma Sintering. Ceram. Int. 2011, 37, 1631–1636. [Google Scholar] [CrossRef]
- Umino, K.; Wakayama, S.; Sakai, T.; Umehara, Y.; Akatsu, T. Mechanical Properties of CNF Reinforced Ceramic Composites Sintered with SPS Technique. J. Solid Mech. Mater. Eng. 2011, 5, 866–872. [Google Scholar] [CrossRef][Green Version]
- Ivanov, R.; Hussainova, I.; Aghayan, M.; Drozdova, M.; Rodríguez, M.A.; Rubio-Marcos, F. Graphene-encapsulated aluminium oxide nanofibers as a novel type of nanofillers for electroconductive ceramics. J. Eur. Ceram. Soc. 2015, 35, 4017–4021. [Google Scholar] [CrossRef]
- Drozdova, M.; Hussainova, I.; Pérez-Coll, D.; Aghayan, M.; Ivanov, R.; Rodríguez, M.A. A novel approach to electroconductive ceramics filled by graphene covered nanofibers. Mater. Des. 2016, 90, 291–298. [Google Scholar] [CrossRef]
- Saheb, N.; Hayat, U. Electrical conductivity and thermal properties and of spark plasma sintered Al2O3-SiC-CNTs hybrid nanocomposites. Ceram. Int. 2017, 43, 5715–5722. [Google Scholar] [CrossRef]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Al-Aqeeli, N.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark plasma sintering of metals and metal matrix nanocomposites: A review. J. Nanomater. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, Z.F.; Lu, J.F.; Shen, X.B.; Wang, F.C.; Wang, Y.D. The sintering mechanism in spark plasma sinteringProof of the occurrence of spark discharge. Scr. Mater. 2014, 81, 56–59. [Google Scholar] [CrossRef]
- Munir, Z.A.; Tamb-urini, U.A.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Orrù, R.; Licheri, R.; Locci, A.M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater. Sci. Eng. R 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Grasso, S.; Sakka, Y.; Maizza, G. Electric current activated/assisted sintering (ECAS): A review of patents 1906–2008. Sci. Technol. Adv. Mater. 2009, 10, 053001. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, D.M.; Anders, A.; Andersson, J.; Lavernia, E.J.; Mukherjee, A.K. A discussion on the absence of plasma in spark plasma sintering. Scr. Mater. 2009, 60, 835–838. [Google Scholar] [CrossRef]
- Kieback, B. A review of spark plasma sintering. In Proceedings of the Hagen Symposium, Hagen, Germany, 24–25 November 2011. [Google Scholar]
- Guyot, P.; Rat, V.; Coudert, J.F.; Jay, F.; Maître, A.; Pradeilles, N. Does the Branly effect occur in spark plasma sintering? J. Phys. D Appl. Phys. 2012, 5, 092001. [Google Scholar] [CrossRef]
- Chaim, R. Densification mechanisms in spark plasma sintering of nanocrystalline ceramics. Mater. Sci. Eng. A 2007, 443, 25–32. [Google Scholar] [CrossRef]
- Chaim, R. On densification mechanisms of ceramic particles during spark plasma sintering. Scr. Mater. 2016, 115, 84–86. [Google Scholar] [CrossRef]
- Aman, Y.; Garnier, V.; Djurado, E. Spark plasma sintering kinetics of pure α-alumina. J. Am. Ceram. Soc. 2011, 94, 2825–2833. [Google Scholar] [CrossRef]
- Chakravarty, D.; Chokshi, A.H. Direct characterizing of densification mechanisms during spark plasma sintering. J. Am. Ceram. Soc. 2014, 97, 765–771. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Z.; Cheng, L. Sintering kinetics window: An approach to the densification process during the preparation of transparent alumina. Adv. Appl. Ceram. 2015, 114, 33–38. [Google Scholar] [CrossRef]
- Hitchcock, D.; Livingston, R.; Liebenberg, D. Improved understanding of the spark plasma sintering process. J. Appl. Phys. 2015, 117, 174505. [Google Scholar] [CrossRef]
- Garay, J.E. Current-Activated, Pressure-Assisted Densification of Materials. Ann. Rev. Mater. Res. 2010, 40, 445–468. [Google Scholar] [CrossRef]
- Van Lier, G.; van Alsenoy, C.; van Doren, V.; Geerlings, P. Ab initio study of the elastic properties of single-walled carbon nanotubes and grapheme. Chem. Phys. Lett. 2000, 326, 181–185. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 37–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically Strong, Electrically Conductive, and Biocompatible Graphene Paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Li, D.; Mueller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Frank, I.W.; Tanenbaum, D.M.; van der Zande, A.M.; McEuen, P.L. Mechanical Properties of Suspended Graphene Sheets. J. Vac. Sci. Technol. B 2007, 25, 2558–2561. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, L.; Fan, F.; Zeng, Z.; Peng, C.; Loya, P.E.; Liu, Z.; Gong, Y.; Zhang, J.; Zhang, X.; et al. Fracture toughness of graphene. Nat. Commun. 2012, 5, 3782. [Google Scholar] [CrossRef] [PubMed]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline ropes of metallic carbon nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Zhaoa, X.; Shimoyama, H.; Sakai, G.; Kaneto, K. Physical properties of multiwalled carbon nanotubes. Int. J. Inorg. Mater. 1999, 1, 77–82. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Deshpande, V.V.; Postma, H.W.C.; Lau, C.N.; Miko, C.; Forro, L.; Bockrath, M. Ballistic Phonon Thermal Transport in Multiwalled Carbon Nanotubes. Phys. Rev. Lett. 2005, 95, 226101. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Shi, L.; Yao, Z.; Li, D.Y.; Majumdar, A. Thermal Conductance and Thermopower of an Individual Single-Wall Carbon Nanotube. Nano Lett. 2005, 5, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2011, 87, 215502. [Google Scholar] [CrossRef] [PubMed]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Wollmershauser, J.A.; Feigelson, B.N.; Gorzkowski, E.P.; Ellis, C.T.; Goswami, R.; Qadri, S.B.; Tischler, J.G.; Kub, F.J.; Everett, R.K. An extended hardness limit in bulk nanoceramics. Acta Mater. 2014, 69, 9–16. [Google Scholar] [CrossRef]
- Pande, C.S.; Cooper, K.P. Nanomechanics of Hall-Petch relationship in nanocrystalline materials. Progr. Mater. Sci. 2009, 54, 689–706. [Google Scholar] [CrossRef]
- ASTM. E384 Standard Test Method for Knoop and Vickers Hardness of Materials; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM. C1161 Standard Test Method for Flexural Strength of Advanced Ceramics at Ambient Temperature; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Lawn, B. Fracture of Brittle Solids, 2nd ed.; Cambridge Solid State Science Series; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Bengisu, M. Properties of Ceramic Materials and Their Evaluation. In Engineering Ceramics; Springer: Berlin, Germany, 2001; pp. 209–362. [Google Scholar]
- Ahmad, I.; Islam, M.; Subhani, T.; Zhu, Y. Toughness enhancement in graphene nanoplatelet/SiC reinforced Al2O3 ceramic hybrid nanocomposites. Nanotechnology 2016, 27, 425704. [Google Scholar] [CrossRef] [PubMed]
- Michálek, M.; Kasiarováˇ, M.; Michálková, M.; Galusek, D. Mechanical and functional properties of Al2O3-ZrO2-MWCNTs Nanocomposites. J. Eur. Ceram. Soc. 2014, 34, 3329–3337. [Google Scholar] [CrossRef]
- Xuefei, L.; Hanlian, L.; Chuanzhen, H.; Bin, Z.; Longwei, Z. High temperature mechanical properties of Al2O3-based ceramic tool material toughened by SiC whiskers and nanoparticles. Ceram. Int. 2017, 43, 1160–1165. [Google Scholar] [CrossRef]
- Anstis, G.R.; Chantikul, P.; Lawn, B.R. A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: I, Direct Crack Measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Chantikul, P.; Anstis, G.R.; Marshall, D.B. A critical evaluation of indentation technique for measuring fracture toughness: II, strength method. J. Am. Ceram. Soc. 1981, 64, 539–543. [Google Scholar] [CrossRef]
- ASTM E399. Standard Test Method for Plain Strain Fracture Toughness of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 1991. [Google Scholar]
- ASTM C1421. Standard Test Methods for Determination of Fracture Toughness of Advanced Ceramics at Ambient Temperature; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Sasaki, S.; Pethic, J.B. Effects of surrounding atmosphere on micro-hardness and tribological properties of sintered alumina. Wear 2000, 241, 204–208. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Baig, Z.; Mamat, O.; Mustapha, M. Recent Progress on the Dispersion and the Strengthening Effect of Carbon Nanotubes and Graphene-Reinforced Metal Nanocomposites: A Review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 1–4. [Google Scholar] [CrossRef]
- Zhang, R.; Baxendale, M.; Peijs, T. Universal resistivity-strain dependence of carbon nanotube/polymer composites. Phys. Rev. B 2007, 76, 195433. [Google Scholar] [CrossRef]
- Kyrylyuk, A.V.; van der Schoot, P. Continuum percolation of carbon nanotubes in polymeric and colloidal media. Proc. Natl. Acad. Sci. USA 2008, 105, 8221–8226. [Google Scholar] [CrossRef] [PubMed]
- Alig, I.; Poetschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]
- Anithambigai, P.; Mutharasu, D.; Huong, L.H.; Zahner, T.; Lacey, D. Synthesis and thermal analysis of aluminium nitride filled epoxy composites and its effective application as thermal interface material for LED applications. J. Mater. Sci. Mater. Electron. 2014, 25, 4814–4821. [Google Scholar] [CrossRef]
- Santanach, J.G.; Weibel, a.; Estourns, C.; Yang, Q.; Laurent, C.; Peigney, a. Spark plasma sintering of alumina: Study of parameters, formal sintering analysis and hypotheses on the mechanism(s) involved in densification and grain growth. Acta Mater. 2011, 59, 1400–1408. [Google Scholar] [CrossRef]
- Shen, Z.; Johnsson, M.; Zhao, Z.; Nygren, M. Spark Plasma Sintering of Alumina. J. Am. Ceram. Soc. 2002, 187381, 1921–1927. [Google Scholar] [CrossRef]
- Wang, S.W.; Chen, L.D.; Hirai, T. Densification of Al2O3 Powder Using Spark Plasma Sintering. J. Mater. Res. 2000, 15, 982–987. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Yang, F.; Chen, K.; An, L. Grain refining in spark plasma sintering Al2O3 ceramics. J. Alloy. Compd. 2015, 622, 596–600. [Google Scholar] [CrossRef]
- Chakravarty, D.; Bysakh, S.; Muraleedharan, K.; Rao, T.N.; Sundaresan, R. Spark plasma sintering of magnesia-doped alumina with high hardness and fracture toughness. J. Am. Ceram. Soc. 2008, 22948, 203–208. [Google Scholar] [CrossRef]
- Zhan, G.D.; Kuntz, J.; Wan, J.; Garay, J.; Mukherjee, A.K. Alumina-based nanocomposites consolidated by spark plasma sintering. Scr. Mater. 2002, 47, 737–741. [Google Scholar] [CrossRef]
- Demuynck, M.; Erauw, J.; van der Biest, O.; Delannay, F.; Cambier, F. Densification of alumina by SPS and HP: A comparative study. J. Eur. Ceram. Soc. 2012, 32, 1957–1964. [Google Scholar] [CrossRef]
- Porwal, H.; Tatarko, P.; Grasso, S.; Khaliq, J.; Dlouhý, I.; Reece, M.J. Graphene reinforced alumina nano-composites. Carbon 2013, 64, 359–369. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.; Jiang, K. Mechanical properties of graphene platelet-reinforced alumina ceramic composites. Ceram. Int. 2013, 39, 6215–6221. [Google Scholar] [CrossRef]
- Morales-Rodriguez, A.; Poyato, R.; Gallardo-lo, A.; Mun, A. Evidence of nanograin cluster coalescence in spark plasma sintered α-Al 2O3. Scr. Mater. 2013, 69, 529–532. [Google Scholar] [CrossRef]
- Zhan, G.D.; Kuntz, J.; Wan, J.; Garay, J.; Mukherjee, A.K. Spark-plasma-sintered BaTiO3/Al2O3 nanocomposites. Mater. Sci. Eng. A 2003, 356, 443–446. [Google Scholar] [CrossRef]
- Jayaseelan, D.D.; Ueno, S.; Ohji, T.; Kanzaki, S. Differential Sintering by Improper Selection of Sintering Parameters during Pulse Electric Current Sintering. J. Am. Ceram. Soc. 2004, 87, 159–161. [Google Scholar] [CrossRef]
- Zhou, Y.; Hirao, K.; Yamauchi, Y.; Kanzaki, S. Densification and grain growth in pulse electric current sintering of alumina. J. Eur. Ceram. Soc. 2004, 24, 3465–3470. [Google Scholar] [CrossRef]
- Morales–Rodríguez, A.; Gallardo–López, A.; Fernández–Serrano, A.; Poyato, R.; Muñoz, A.; Domínguez–Rodríguez, A. Improvement of Vickers hardness measurement on SWNT/Al2O3 composites consolidated by spark plasma sintering. J. Eur. Ceram. Soc. 2014, 34, 3801–3809. [Google Scholar] [CrossRef]
- Meng, S.; Guo-Hong, Z.; Shi-Wei, W. Fabrication of Sub-micron Alumina by Spark Plasma Sintering. J. Inorg. Mater. 2008, 23, 3–6. [Google Scholar]
- Kasperski, A.; Weibel, A.; Estournès, C.; Laurent, C.; Peigney, a. Preparation-microstructure-property relationships in double-walled carbon nanotubes/alumina composites. Carbon 2013, 53, 62–72. [Google Scholar] [CrossRef]
- Hirota, K.; Takaura, Y.; Kato, M.; Miyamoto, Y. Fabrication of carbon nanofiber(CNF)-dispersed Al2O3 composites by pulsed electric-current pressure sintering and their mechanical and electrical properties. J. Mater. Sci. 2007, 42, 4792–4800. [Google Scholar] [CrossRef]
- Álvarez-Clemares, I.; Borrell, A.; Agouram, S.; Torrecillas, R.; Fernández, a. Microstructure and mechanical effects of spark plasma sintering in alumina monolithic ceramics. Scr. Mater. 2013, 68, 603–606. [Google Scholar] [CrossRef]
- Zhan, G.D.; Mukherjee, A.K. Carbon Nanotube Reinforced alumina based ceramics with novel electrical, mechanical and thermal properties. Int. J. Appl. Ceram. Technol. 2004, 1, 161–171. [Google Scholar] [CrossRef]
- Ahmad, K.; Wei, P.; Wan, C. Thermal conductivities of alumina-based multiwall carbon nanotube ceramic composites. J. Mater. Sci. 2014, 49, 6048–6055. [Google Scholar] [CrossRef]
- Saheb, N.; Hayat, U. Temperature-dependent thermal properties of spark plasma sintered alumina. Sci. Sinter. 2017, 49, 117–128. [Google Scholar] [CrossRef]
- Saheb, N.; Mohammad, K. Hard and Tough Al2O3-SiC-CNT Hybrid Ceramic Nanocomposite Produced by Molecular Level Mixing and Spark Plasma Sintering. J. Aust. Ceram. Soc. 2018, 54, 401–410. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Chen, Z.; Wyszomirska, M.; Zhao, J.; Jiang, Z. Microstructure and tribological behaviour of alumina composites reinforced with SiC-graphene core-shell nanoparticles. Tribol. Int. 2019, 131, 94–101. [Google Scholar] [CrossRef]
- Rahman, O.S.A.; Sribalaji, M.; Mukherjee, B.; Laha, T.; Keshri, A.K. Synergistic effect of hybrid carbon nanotube and graphene nanoplatelets reinforcement on processing, microstructure, interfacial stress and mechanical properties of Al2O3 nanocomposites. Ceram. Int. 2018, 44, 2109–2122. [Google Scholar] [CrossRef]
- Wozniak, J.; Cygan, T.; Petrus, M.; Cygan, S.; Kostecki, M.; Jaworska, L.; Olszyna, A. Tribological performance of alumina matrix composites reinforced with nickel-coated graphene. Ceram. Int. 2018, 44, 9728–9732. [Google Scholar] [CrossRef]
- Petrus, M.; Wozniak, J.; Cygan, T.; Kostecki, M.; Cygan, S.; Jaworska, L.; Teklińska, D.; Olszyna, A. Comprehensive study on graphene-based reinforcements in Al2O3–ZrO2 and Al2O3–Ti (C, N) systems and their effect on mechanical and tribological properties. Ceram. Int. 2018, 44, 9728–9732. [Google Scholar] [CrossRef]
- Xia, Z.; Riester, L.; Curtin, W.A.; Li, H.; Sheldon, B.W.; Liang, J.; Chang, B.; Xu, J.M. Direct observation of toughening mechanisms in carbon nanotube ceramic matrix composites. Acta Mater. 2004, 52, 931–944. [Google Scholar] [CrossRef]
- Xia, Z.; Curtin, W.A.; Sheldon, B.W. Fracture toughness of highly ordered carbon nanotube/alumina nanocomposites. J. Eng. Mater. Technol. Trans. Asme 2004, 126, 238–244. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, J.F.; Zhang, B.P.; Yao, K.F.; Liu, W.S.; Wang, H. Electrical and Thermal Properties of Carbon Nanotube Bulk Materials: Experimental Studies for the 328–958 K Temperature Range. Phys. Rev. B 2007, 75, 1–2. [Google Scholar] [CrossRef]
- Qin, C.; Shi, X.; Bai, S.Q.; Chen, L.D.; Wang, L.J. High Temperature Electrical and Thermal Properties of the Bulk Carbon Nanotube Prepared by SPS. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2006, 420, 208–211. [Google Scholar] [CrossRef]
- Gong, J.; Zhao, Z.; Miao, H.; Guan, Z. R-Curve behavior of TiC particle reinforced Al2O3 composites. Scr. Mater. 2000, 43, 27–31. [Google Scholar] [CrossRef]
- Gong, J.; Zhao, Z.; Guan, Z. On the local crack resistance of Al2O3–TiC composites evaluated by direct indentation method. J. Eur. Ceram. Soc. 2001, 21, 941–946. [Google Scholar] [CrossRef]
- Siddiqui, M.U.; Hayat, U.; Arif, A.F.M.; Saheb, N. On the Thermal Conductivity of Spark Plasma Sintered Alumina Hybrid Nanocomposites: Estimation Modeling and Experimental Validation. Sci. Sinter. 2019, 51, 101–114. [Google Scholar] [CrossRef]
- Hayashi, T.; Endo, M. Carbon nanotubes as structural material and their application in composites. Compos. B 2011, 42, 2151–2157. [Google Scholar] [CrossRef]
- Ahmad, I.; Unwin, M.; Cao, H.; Chen, H.; Zhao, H.; Kennedy, A.; Zhu, Y.Q. Multi-walled carbon nanotubes reinforced Al2O3 nanocomposites: Mechanical properties and interfacial investigations. Comp. Sci. Technol. 2010, 70, 1199–1206. [Google Scholar] [CrossRef]
- Vasiliev, A.L.; Poyato, R.; Padture, N.P. Single-wall carbon nanotubes at ceramic grain boundaries. Scr. Mater. 2007, 56, 461–463. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, L.; Sun, J. Carbon nanotube-ceramic composites. J. Electroceram. 2006, 17, 51–55. [Google Scholar] [CrossRef]
- Wade, J.; Wu, H. Hardness of alumina/silicon carbide nanocomposites at various silicon carbide volume percentages. In Ceramic Engineering and Science Proceedings; Mathur, S., Ed.; Nanostructured Materials and Nanotechnology VII; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 34, pp. 119–130. [Google Scholar]
- Wana, Y.; Gong, J.H. Influence of TiC particle size on the load-independent hardness of Al2O3–TiC composites. Mater. Lett. 2003, 57, 3439–3443. [Google Scholar] [CrossRef]
- Ahmad, I.; Islam, M.; Parvez, S.; AlHabis, N.; Umar, A.; Munir, K.S.; Wang, N.; Zhu, Y. Reinforcing capability of multiwall carbon nanotubes in alumina ceramic hybrid nanocomposites containing zirconium oxide nanoparticles. Int. J. Refract. Met. Hard Mater. 2019, 84, 105018. [Google Scholar] [CrossRef]
- Iftikhar, A.; Islam, M. Reinforcing ability and bonding characteristics of multiwall carbon nanotubes and silicon carbide nanoparticles in inductively sintered alumina ceramic hybrid nanocomposites. J. Alloy. Compd. 2019, 788, 219–230. [Google Scholar]
- Iftikhar, A.; Parvez, S.; Saeed, K. Interfacial investigation, mechanical performance and thermal permanence of the inductively hot-pressed alumina ceramic hybrid nanocomposites reinforced by silicon carbide and multilayer graphene. Int. J. Refract. Met. Hard Mater. 2019, 81, 49–57. [Google Scholar]
- Singh, P.; Chauhan, N.R.; Rajesha, S. Influence of cobalt, iron and copper on microstructure and mechanical properties of alumina/SiC nano-ceramic composite. Mater. Res. Express 2019, 6, 065027. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. Apl Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Fischer, F. Research Features. Available online:www.researchfeatures.com (accessed on 05 May 2019).


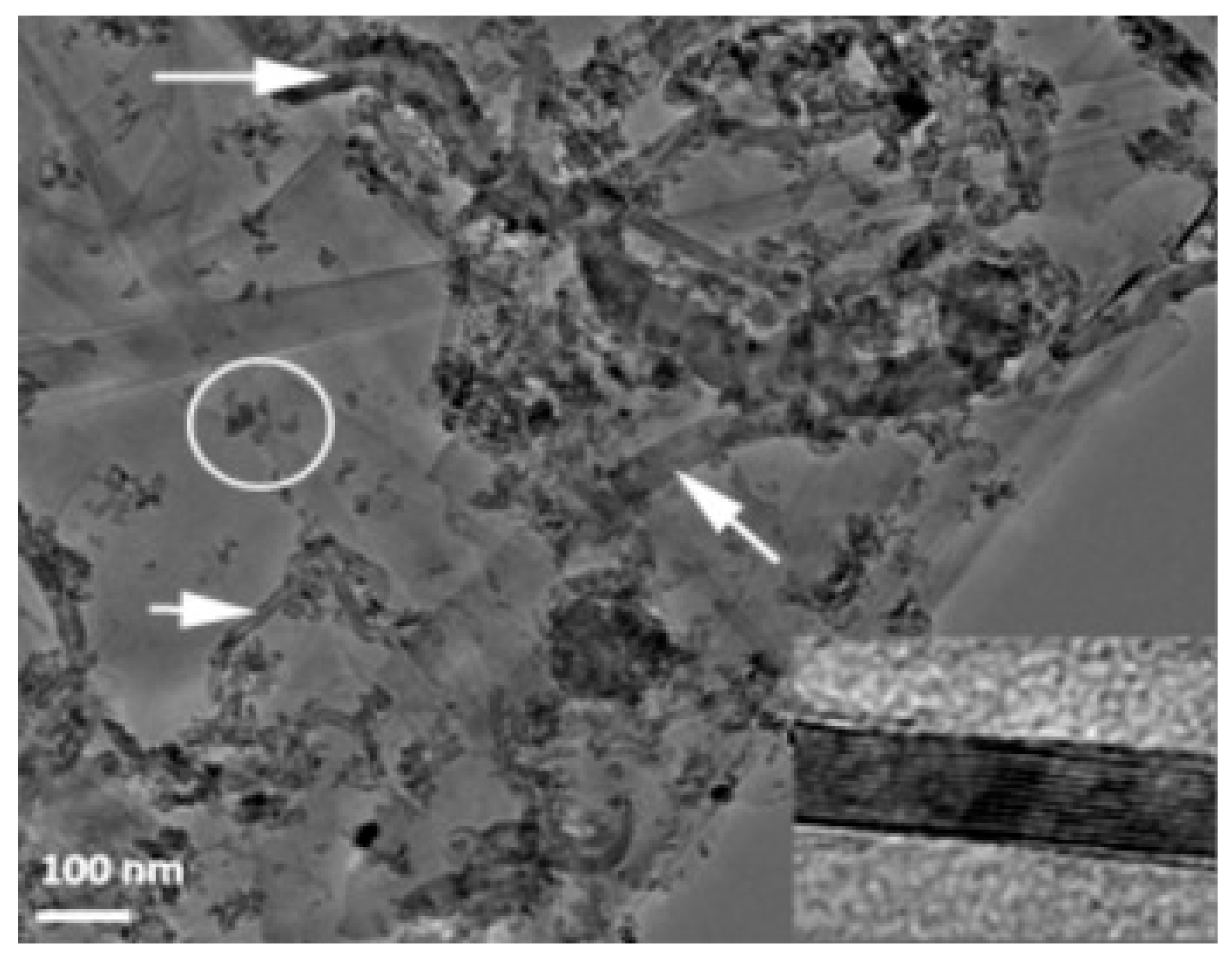


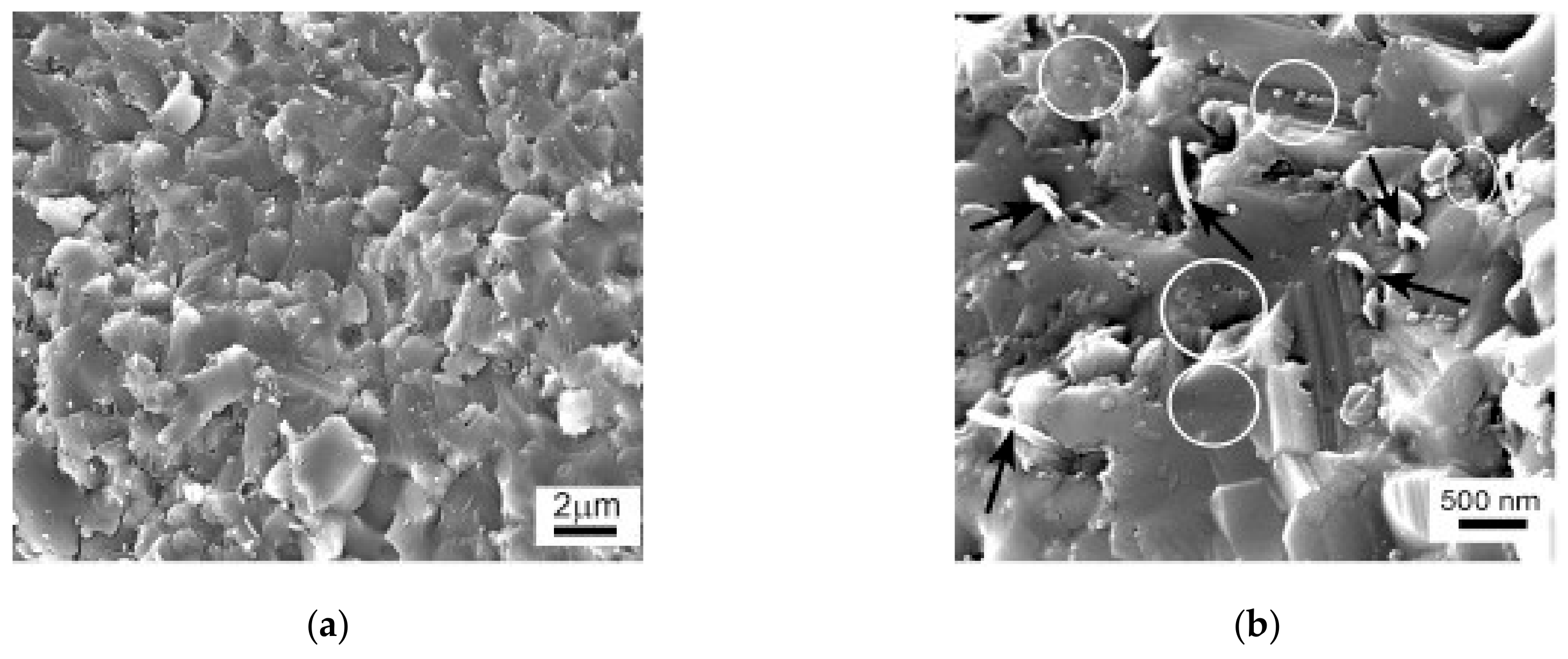

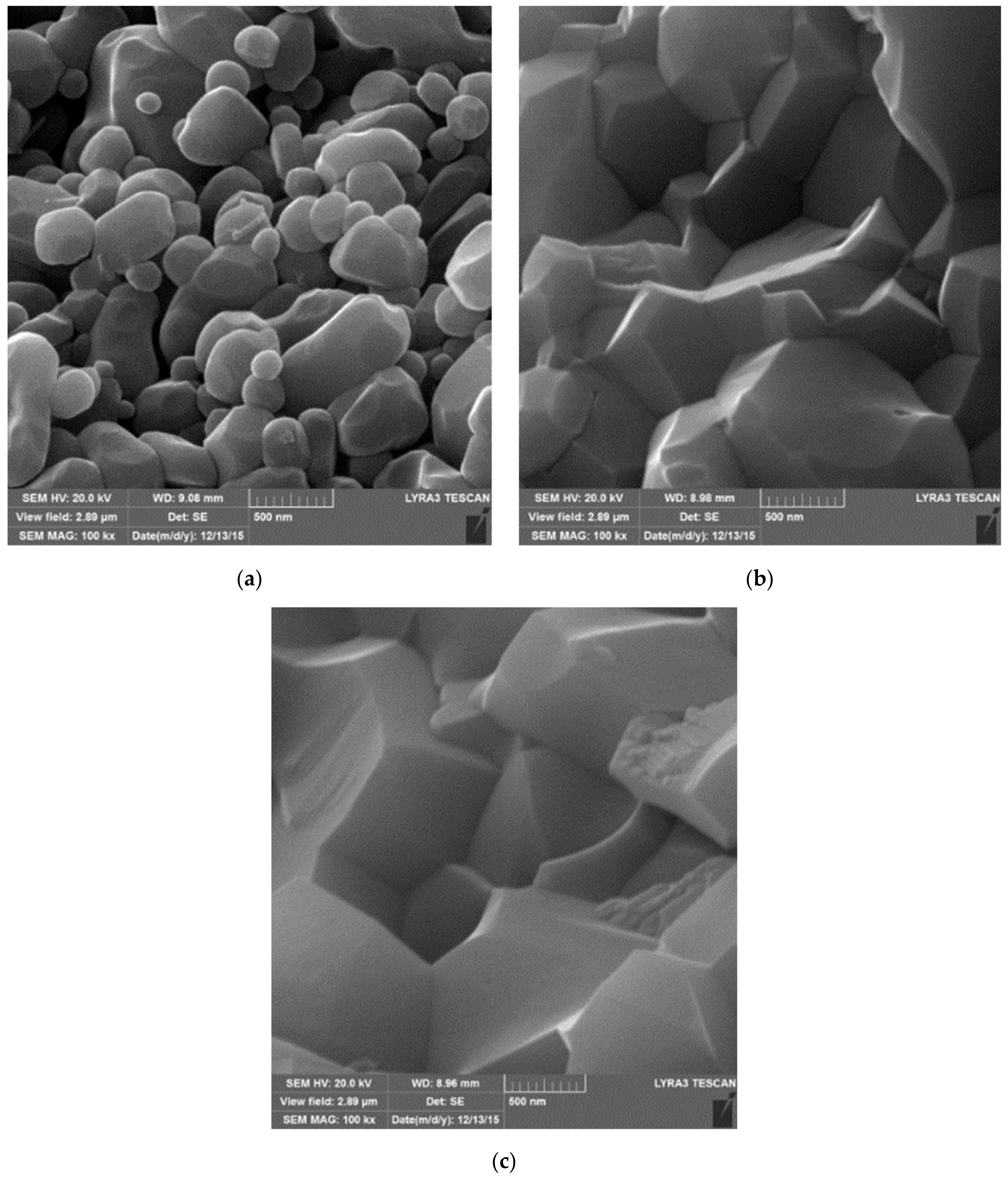
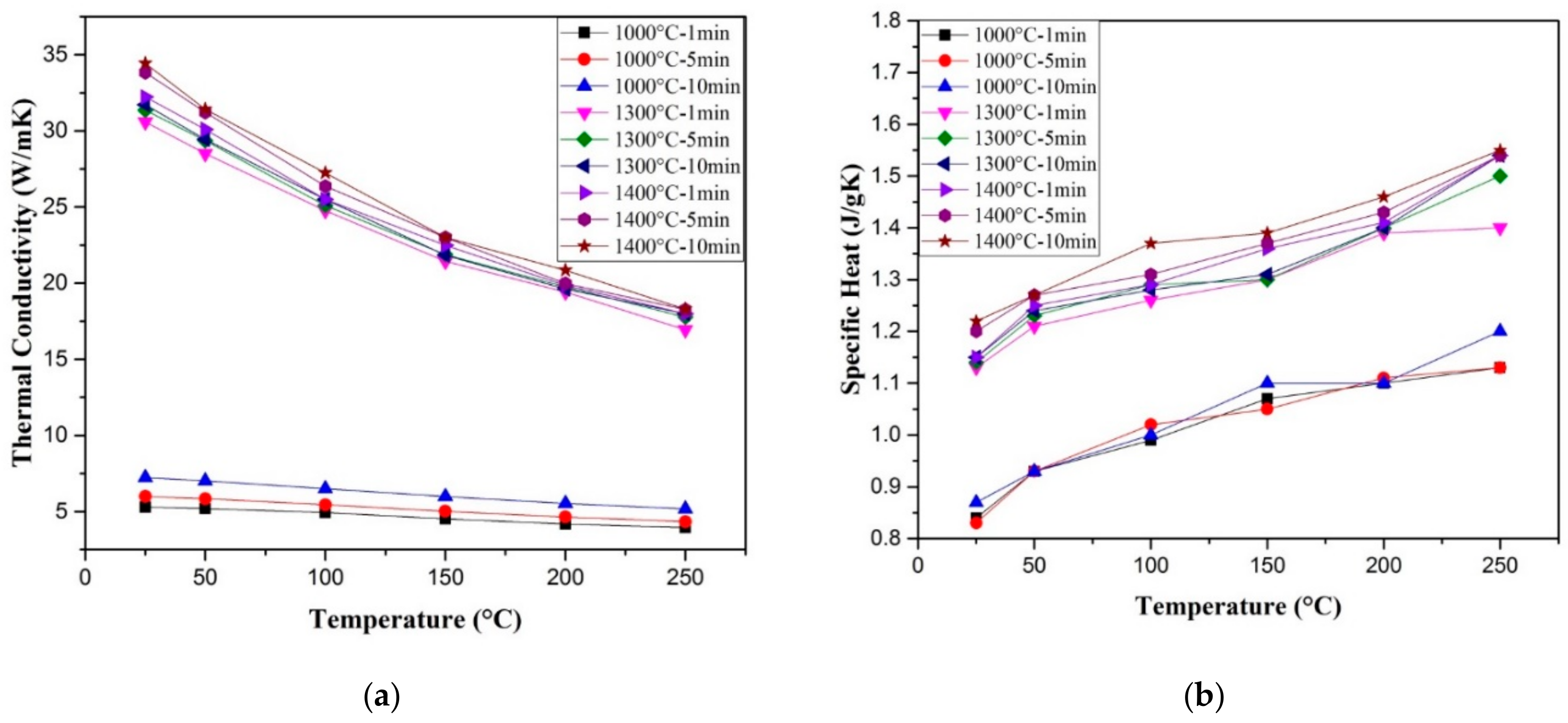












| Sample | Density (%) | Electrical Conductivity (S/cm) | Grain Size (μm) | Vickers Hardness (GPa) | Fracture Toughness (MPa∙m0.5) |
|---|---|---|---|---|---|
| Al2O3 (AO) | >99.0 | 8.92 ± 3.86 × 10−13 | 5.76 ± 2.38 | 15.77 ± 0.71 | 3.30 ± 0.12 |
| Al2O3-5SiC (AS) | >99.0 | 1.49 ± 0.29 × 10−7 | 1.24 ± 0.38 | 19.43 ± 0.65 | 3.17 ± 0.14 |
| Al2O3-5SiC-0.5GNSs (ASG1) | >99.0 | 3.62 ± 1.62 × 10−7 | 1.26 ± 0.43 | 18.07 ± 0.47 | 3.51 ± 0.09 |
| Al2O3-5SiC-1GNSs (ASG2) | >99.0 | 1.86 ± 0.03 | 1.23 ± 0.45 | 17.07 ± 0.26 | 3.92 ± 0.22 |
| Sample Composition | Experimental Sample Thermal Conductivity [W/m·K] | Predicted Results with Crystallite Size Effect [W/m·K] | Predicted Results without Crystallite Size Effect [W/m·K] |
|---|---|---|---|
| Al2O3 | 34.44 | 34.06 | 33.92 |
| Al2O3-5SiC-1CNT | 22.20 | 27.60 | 29.99 |
| Al2O3-5SiC-2CNT | 21.33 | 25.60 | 29.34 |
| Al2O3-10SiC-1CNT | 17.75 | 21.38 | 26.69 |
| Al2O3-10SiC-2CNT | 17.83 | 19.62 | 26.30 |
| Materials Composition (%) | SPS Process Parameters | Microstructure Characteristics | Mechanical Properties | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | First Reinforcement | Second Reinforcement | Heating Rate (deg./min.) | Pressure (MPa) | Temperature (°C) | Time (min) | Relative Density (%) | Alumina Grain Size (μm) | Hardness (MPa) | Fracture Toughness (MPa m 1/2) | Flexural Strength (MPa) | - |
| 100 | - | - | - | 50 | 1550 | - | 100 | - | ˃17 | ˃3 | ˃335 | [32] |
| Bal. | 1vol.%SiC | 5wt.%CNTs | - | 50 | 1550 | - | 98.2 | - | ˃16 | ˃6 | ˃485 | [32] |
| Bal. | 1vol.%SiC | 7wt.%CNTs | - | 50 | 1550 | - | 97.2 | - | ˃15 | ˃6 | ˃480 | [32] |
| Bal. | 1vol.%SiC | 10wt.%CNTs | - | 50 | 1550 | - | 95.1 | - | ˃14 | ˃5 | ˃440 | [32] |
| 100 | - | - | - | 50 | 1550 | - | 100 | - | 17 | ˂4 | ˃330 | [40] |
| Bal. | 1vol.%SiC | 5vol.%CNTs | - | 50 | 1550 | - | 98 | - | 17 | ˃6 | ≈500 | [40] |
| Bal. | 2vol.%SiC | 5vol.%CNTs | - | 50 | 1550 | - | 96.46 | - | 16 | ≈6 | ≈450 | [40] |
| Bal. | 3vol.%SiC | 5vol.%CNTs | - | 50 | 1550 | - | 96.40 | - | 16 | ˂6 | ≈450 | [40] |
| 100 | - | - | 100 | 50 | 1500 | 10 | 99.3 | - | 18.56 | 3.61 | - | [41] |
| Bal. | 5wt.%SiC | 1wt.%CNTs | 100 | 50 | 1500 | 10 | 99.36 | - | 19.77 | 3.89 | - | [41] |
| Bal. | 5wt.%SiC | 2wt.%CNTs | 100 | 50 | 1500 | 10 | 98.28 | - | 19.11 | 4.2 | - | [41] |
| Bal. | 10wt.%SiC | 1wt.%CNTs | 100 | 50 | 1500 | 10 | 98.63 | - | 20.81 | 4.58 | - | [41] |
| Bal. | 10wt.%SiC | 2wt.%CNTs | 100 | 50 | 1500 | 10 | 98.02 | - | 17.50 | 6.98 | - | [41] |
| 100 | - | - | 100 | 50 | 1500 | 10 | 99.3 | 18.56 | 3.61 | - | [43] | |
| Bal. | 5wt.%SiC | 1wt.%CNTs | 100 | 50 | 1500 | 10 | 91.65 | 17.81 | 5.83 | - | [43] | |
| 100 | - | - | 100 | 50 | 1500 | 3 | 100 | 4.68 | 18.04 | 3.53 | 400 | [39] |
| Bal. | 1vol.%SiC | 0.38vol.%GPL | 100 | 50 | 1500 | 3 | 99.03 | 3.67 | 21.34 | 4.77 | 572 | [39] |
| Bal. | 3vol.%SiC | 0.38vol.%GPL | 100 | 50 | 1500 | 3 | 98.85 | 2.66 | 24.65 | 5.03 | 520 | [39] |
| Bal. | 5vol.%SiC | 0.38vol.%GPL | 100 | 50 | 1500 | 3 | 97.35 | 2.33 | 21.58 | 4.94 | 535 | [39] |
| 100 | - | - | 100 | 40 | 1650 | 10 | 98 | 12 | 13.5 | ≈4 | 350 | [53] |
| 0.5wt.%GNT | 0.5wt.%GNT | 100 | 40 | 1650 | 10 | 99 | - | 14.75 | 5.75 | 450 | [53] | |
| Bal. | 0.5wt.%GNT | 1wt.%CNT | 100 | 40 | 1650 | 10 | 99 | - | 15.5 | ≈4.5 | 325 | [53] |
| Bal. | 1wt.%GNT | 1wt.%CNT | 100 | 40 | 1650 | 10 | 98 | - | 11.20 | ≈4 | 340 | [53] |
| Bal. | xvol.%SiCW | - | 25 | 30 | 1780 | 15 | 95.53 | - | 15.85 | - | 525 | [54] |
| Bal. | xvol.%SiCW | 22vol.%TiC | 25 | 30 | 1780 | 15 | 99.74 | - | 21.60 | - | 648 | [54] |
| 42 | 36%SiCW | 22%TiC | 100 | 40 | 1780 | 5 | ˃99 | - | 22.74 | 6.5 | - | [55] |
| 100 | - | - | 50 | 100 | 1375 | 3 | >98 | 3 ± 1 | 19.9 | 3.5 | 395 | [56] |
| 73.1 | 25vol.% nTiC | 1.9vol.% nNi | 50 | 100 | 1375 | 3 | >98 | 0.3 ± 0.1 | 25.6 | 3.7 | 537 | [56] |
| 70 | 20vol.%CNF | 10vol.%SiC | - | 50 | 1500 | 3 | - | - | - | 2.79 | 144 | [57] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saheb, N.; Hayat, U.; Hassan, S.F. Recent Advances and Future Prospects in Spark Plasma Sintered Alumina Hybrid Nanocomposites. Nanomaterials 2019, 9, 1607. https://doi.org/10.3390/nano9111607
Saheb N, Hayat U, Hassan SF. Recent Advances and Future Prospects in Spark Plasma Sintered Alumina Hybrid Nanocomposites. Nanomaterials. 2019; 9(11):1607. https://doi.org/10.3390/nano9111607
Chicago/Turabian StyleSaheb, Nouari, Umer Hayat, and Syed Fida Hassan. 2019. "Recent Advances and Future Prospects in Spark Plasma Sintered Alumina Hybrid Nanocomposites" Nanomaterials 9, no. 11: 1607. https://doi.org/10.3390/nano9111607
APA StyleSaheb, N., Hayat, U., & Hassan, S. F. (2019). Recent Advances and Future Prospects in Spark Plasma Sintered Alumina Hybrid Nanocomposites. Nanomaterials, 9(11), 1607. https://doi.org/10.3390/nano9111607






