Nanoparticulate Metal Oxide Top Electrode Interface Modification Improves the Thermal Stability of Inverted Perovskite Photovoltaics
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of γ-Fe2O3 NPs
2.3. Preparation of Ligand-Stripped γ-Fe2O3 NPs
2.4. Device Fabrication
2.5. Characterization
3. Results and Discussion
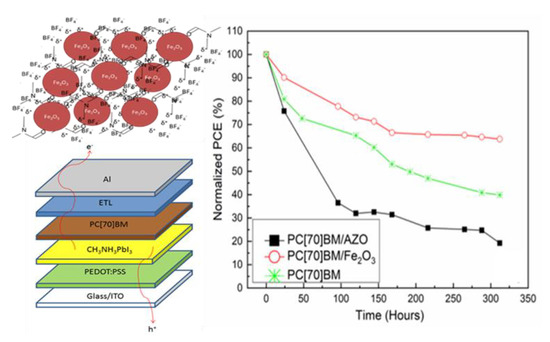
3.1. γ-Fe2O3 Interface Modification for Inverted Perovskite Solar Cells
3.2. Inverted Perovskite Solar Cells Heat Accelerated Lifetime Studies
3.3. Device Performance Analysis and Degradation Mechanisms
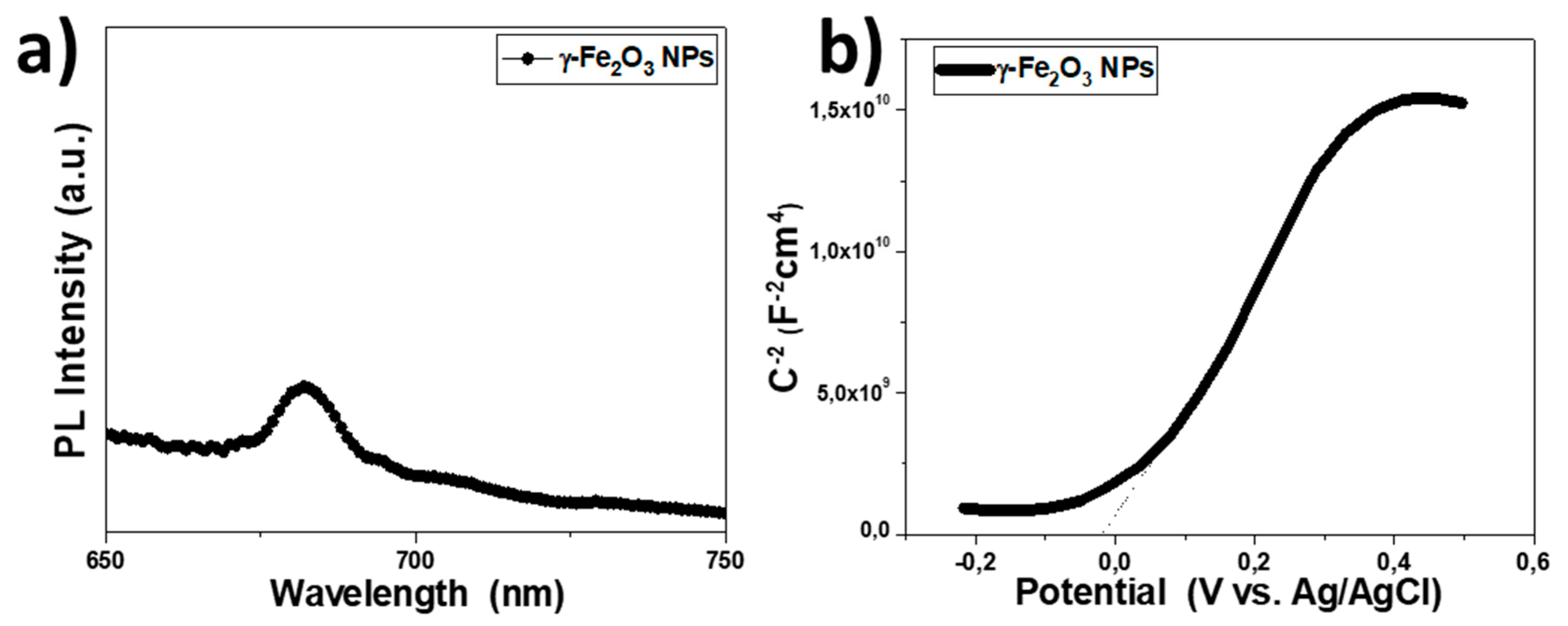
3.4. Impedance Spectroscopy Device Characterization and the Effect of Interfacial Trap States
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasak, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Duong, T.; Wu, Y.; Peng, Y.; Jacobs, D.; Wu, N.; Weber, K.; White, T.; Catchpole, K. Metal Halide Perovskite: A Game-Changer for Photovoltaics and Solar Devices via a Tandem Design. Sci. Technol. Adv. Mater. 2018, 19, 53–71. [Google Scholar] [CrossRef]
- Wehrenfenning, C.; Eperon, G.E.; Johnston, M.B.; Snaith, M.B.; Herz, L.M. High Charge Carrier Mobilities and Lifetimes in Organolead Trihalide Perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seong, S. Chemical Management for Colorful, Efficient and Stable Inorganic-Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Liu, Y.; Bag, M.; Renna, L.A.; Page, Z.A.; Kim, P.; Emrick, T.; Venkataraman, D.; Russel, T.P. Understanding Interface Engineering for High-Performance Fullerene/Perovskite Planar Heterojunction solar cells. Adv. Energy Mater. 2015, 6, 1501606. [Google Scholar] [CrossRef]
- Savva, A.; Burgues-Ceballos, I.; Choulis, S.A. Improved Performance and Reliability of p-i-n Perovskite Solar Cells via Doped Metal Oxides. Adv. Energy Mater. 2016, 6, 1600285. [Google Scholar] [CrossRef]
- Eperon, G.E.; Strank, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low Cost, High Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Stoumpos, C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with organic cations: Phase Transitions, High Mobilities and Near-Infrared Photoluminescent Properites. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Choi, H.; Mai, C.K.; Kim, H.; Song, S.; Bazan, G.C.; Kim, J.K.; Heeger, A.J. Conjugated Polyelectrolyte Hole Transport Layer for Inverted-Type Perovskite Solar Cells. Nat. Commun. 2015, 6, 8348–8353. [Google Scholar] [CrossRef]
- Cao, J.; Yin, J.; Yuan, S.; Zhao, Y.; Li, J.; Zheng, N. Thiols as Ιnterfacial Modifiers to Enhance the Performance and Stability of Perovskite Solar Cells. Nanoscale 2015, 7, 9443–9447. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarr, D.; McGehe, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. 2014, 126, 11414–11417. [Google Scholar] [CrossRef]
- Leijtnes, T.; Bush, K.; Cheachaoren, R.; Beal, R.; Bowring, A.; McGehee, M.D. Towards Enabling Stable Lead Halide Perovskite Solar Cells; Interplay Between Structural, Environmental and Thermal Stability. J. Mater. Chem. A 2017, 5, 11483–11500. [Google Scholar] [CrossRef]
- Fang, R.; Wu, S.; Chen, W.; Liu, Z.; Zhang, S.; Chen, R.; Yue, Y.; Dong, L.; Cheng, Y.; Han, L.; et al. [6,6]-Phenyl-C61-Butyric Acid Methyl Ester/Cerium Oxide Bilayer Structure as Efficient and Stable Electron Transport Layer for Inverted Perovskite Solar Cells. ACS Nano 2018, 12, 2403–2414. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers. Angew. Chem. Int. Ed. 2015, 54, 8208–8212. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, J.; Arivazhagan, V.; Xiao, K.; Qiang, Y.; Huang, K.; Hu, M.; Cui, C.; Yu, X.; Yang, D. Efficient and Highly Stable Planar Perovskite Solar Cells with Graphene Quantum Dots Doped PCBM Electron Transport Layer. Nano Energy 2017, 40, 345–351. [Google Scholar] [CrossRef]
- Alsari, M.; Pearson, A.J.; Wang, J.T.; Wang, Z.; Montisci, A.; Greenham, N.C.; Snaith, H.J.; Liliu, S.; Friend, R.H. Degradation Kinetics of Inverted Perovskite Solar Cells. Sci. Rep. 2018, 8, 5977. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babaygit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, H.; Seo, J.; Par, N. Effect of Selective Contacts on the Thermal Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 7148–7153. [Google Scholar] [CrossRef]
- Domanski, K.; Correa-Baena, J.; Mine, N.; Nazeeruddin, M.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Gradzel, M. Not all that Glitters is Gold: Metal Migration-Induced Degradation in Perovskite Solar Cells. ACS Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef]
- Mingorance, A.; Xie, H.; Kim, H.; Wang, Z.; Balsells, M.; Morales-Melgares, A.; Domingo, N.; Kazuteru, N.; Tress, W.; Fraxedas, J.; et al. Interfacial Engineering of Metal Oxides for Highly Stable Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800367. [Google Scholar] [CrossRef]
- Brinkmann, K.; Zhao, J.; Pourdavoud, N.; Becker, T.; Hu, T.; Oltho, S.; Meerholz, K.; Hoffmann, L.; Gahlmann, T.; Heiderhoff, R.; et al. Suppressed Decomposition of Organometal Halide Perovskites by Impermeable Electron-Extraction Layers in Inverted Solar Cells. Nat. Commun. 2017, 8, 13938–13946. [Google Scholar] [CrossRef] [PubMed]
- Papadas, I.T.; Savva, A.; Ioakeimidis, A.; Eleftheriou, P.; Armatas, G.S.; Choulis, S.A. Employing Surfactant-Assisted Hydrothermal Synthesis to Control CuGaO2 Nanoparticle Formation and Improved Carrier Selectivity of Perovskite Solar Cells. Mater. Today Energy 2018, 8, 57–64. [Google Scholar] [CrossRef]
- Papadas, I.T.; Ioakeimidis, A.; Armatas, G.S.; Choulis, S.A. Low-Temperature Combustion Synthesis of Spinel NiCo2O4, Hole Transport Layer for Perovskite Photovoltaics. Adv. Sci. 2018, 5, 1701029. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Legros, J.; Paih, J.L.; Zani, L. Iron-Catalyzed Reactions in Organic Synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar] [CrossRef]
- Morris, R.H. Asymmetric Hydrogenation, Transfer Hydrogenation and Hydrosilylation of Ketones Catalyzed by Iron Complexes. Chem. Soc. Rev. 2009, 38, 2282–2291. [Google Scholar] [CrossRef]
- Gopalaiah, K. Chiral Iron Catalysts for Asymmetric Synthesis. Chem. Rev. 2013, 113, 3248–3296. [Google Scholar] [CrossRef]
- Papadas, I.T.; Fountoulaki, S.; Lykakis, I.N.; Armatas, G.S. Controllable Synthesis of Mesoporous Iron Oxide Nanocrystal Assemblies for Chemoselective Nitroarene Transfer Hydrogenation Chemistry. Eur. J. Chem. 2016, 22, 4600–4607. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Yan, G.; Jiang, Y.; Kuang, C.; Wang, S.; Liu, H.; Zhang, Y.; Wang, J. Nano-Fe2O3-Catalyzed Direct Borylation of Arenes. Chem. Commun. 2010, 46, 3170–3172. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Gholinejad, M.; Hoseini, J. Magnetite (Fe3O4) Nanoparticles-Catalyzed Sonogashira-Hagihara Reactions in Ethylene Glycol under Ligand-Free Conditions. Adv. Synth. Catal. 2011, 353, 125–132. [Google Scholar] [CrossRef]
- Rajabi, F.; Karimi, N.; Saidi, M.R.; Primo, A.; Varma, R.S.; Luque, R. Unprecedented Selective Oxidation of Styrene Derivatives using a Supported Iron Oxide Nanocatalyst in Aqueous Medium. Adv. Synth. Catal. 2012, 354, 1707–1711. [Google Scholar] [CrossRef]
- Shi, F.; Tse, M.K.; Pohl, M.M.; Bruckner, A.; Zhang, S.; Beller, M. Tuning Catalytic Activity between Homogeneous and Heterogeneous Catalysis: Improved Activity and Selectivity of Free Nano-Fe2O3 in Selective Oxidations. Angew. Chem. Int. Ed. 2007, 46, 8866–8868. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Sun, Z.; Wu, S.; Qi, W.; Wang, H.; Xu, X.; Su, D. Surfactant-free Hydrothermal Synthesis of Sub-10 nm γ-Fe2O3–Polymer Porous Composites with High Catalytic Activity for Reduction of Nitroarenes. Chem. Commun. 2013, 49, 10088–10090. [Google Scholar] [CrossRef]
- Sivula, K.; Formal, F.L.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–446. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C. Continuous Aspect-Ratio Tuning and Fine Shape Control of Monodisperse α-Fe2O3 Nanocrystals by a Programmed Microwave–Hydrothermal Method. Adv. Funct. Mater. 2008, 18, 880–887. [Google Scholar] [CrossRef]
- Bouhjar, F.; Mollar, M.; Ullah, S.; Mari, B.; Bessairs, B. Influence of a Compact α-Fe2O3 Layer on the Photovoltaic Performance of Perovskite-Based Solar Cell. J. Electrochem. Soc. 2018, 165, 30–38. [Google Scholar] [CrossRef]
- Lao, Q.; Chen, H.; Lin, Y.; Du, H.; Hou, Q.; Hao, F.; Wang, N.; Guo, Z.; Huang, J. Discrete Iron (III) Oxide Nanoislands for Efficient and Photostable Perovskite Solar Cells. Adv. Funct. Mater 2017, 27, 1702090. [Google Scholar] [CrossRef]
- Hou, Q.; Ren, J.; Chen, H.; Yang, P.; Shao, Q.; Zhao, M.; Zhao, X.; He, H.; Wang, N.; Luo, Q.; et al. Synergistic Hematite-Fullerene Electron-Extracting Layers for Improved Efficiency and Stability in Perovskite Solar Cells. ChemElectroChem 2018, 5, 726–731. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Llordes, A.; Hammack, T.; Buonsanti, R.; Tangirala, R.; Aloni, S.; Helms, B.A.; Milliron, D.J. Polyoxometalates and Colloidal Nanocrystals as Building Blocks for Metal Oxide Nanocomposite Films. J. Mater. Chem. 2011, 21, 11631–11638. [Google Scholar] [CrossRef]
- Chen, W.Y.; Deng, L.L.; Dai, S.M.; Wang, X.; Tian, C.B.; Zhan, X.X.; Xie, S.Y.; Huang, R.B.; Zheng, L.S. Low-cost Solution-processed Copper Iodide as an Alternative to PEDOT: PSS Hole Transport Layer for Efficient and Stable Inverted Planar Heterojunction Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 19353–19359. [Google Scholar] [CrossRef]
- Galatopoulos, F.; Papadas, I.T.; Armatas, G.S.; Choulis, S.A. Long Thermal Stability οf Inverted Perovskite Photovoltaics Incorporating Fullerene-Based Diffusion Blocking Layer. Adv. Mater. Interfaces 2018, 5, 1800280. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Yang, M.; Priya, S.; Zhu, K. Origin of J-V Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 905–917. [Google Scholar] [CrossRef]
- Futscher, M.H.; Lee, J.M.; McGovern, L.; Muscarella, L.A.; Wang, T.; Haider, M.I.; Fakharuddin, A.; Schmidt-Mende, L.; Ehrler, B. Quantification of Ion Migration in CH3NH3PbI3 Perovskite Solar Cells by Transient Capacitance Measurements. arXiv 2018, arXiv:1801.08519. [Google Scholar] [CrossRef] [Green Version]
- Bi, E.; Chen, H.; Xie, F.; Wu, Y.; Chen, W.; Su, Y.; Islam, A.; Gratzel, M.; Yang, X.; Han, L. Diffusion Engineering of Ions and Charge Carriers for Stable Efficient Perovskite Solar Cells. Nat. Commun. 2017, 8, 15330–15336. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, A.; You, J.; Aranda, C.; Kang, Y.; Garcia-Belmonte, G.; Zhou, H.; Bisquert, J.; Yang, Y. Interfacial Degradation of Planar Halide Perovskite Solar Cells. ACS Nano 2016, 10, 218–224. [Google Scholar] [CrossRef]
- Tessler, N.; Vaynzof, Y. Preventing Hysteresis in Perovskite Solar Cells by Undoped Charge Blocking Layers. ACS Appl. Energy Mater. 2018, 1, 676–683. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Q.D.; Xiao, J.; Xue, Q.; Li, H.W.; Guan, Z.; Yip, H.L.; Tsang, S.W. Decomposition of Organometal Halide Perovskite Films on Zinc Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19986–19993. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Mosconi, E.; Angelis, F.D.; Kelly, T.L. Origin of the Thermal Instability in CH3NH3PbI3 Thin Films Deposited on ZnO. Chem. Mater. 2015, 27, 4229–4236. [Google Scholar] [CrossRef]
- Haruo, W.; Jun’etsu, S. The Point of Zero Charge and the Isoelectric Point of γ-Fe2O3 and α-Fe2O3. Bull. Chem. Soc. Jpn. 1986, 59, 2683–2687. [Google Scholar]
- Kosmulski, M. Chemical Properties of Material Surfaces; Marcel Dekker: New York, NY, USA, 2001; pp. 1–76. ISBN 9780585418049. [Google Scholar]
- Venn, R.F. Principles and Practice of Bioanalysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–340. ISBN 9780849338571. [Google Scholar]
- Zohar, A.; Kulbak, M.; Levine, I.; Hodes, G.; Kahn, A.; Cahen, D. What Limits the Open-Circuit Voltage of Bromide Perovskite-Based Solar Cells. ACS Energy Lett. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Paach, G.; Sheinert, S. Simulation and modelling of C-V curves of OLEDs with trapped states for the holes. Synth. Met. 2001, 122, 145–147. [Google Scholar] [CrossRef]
- Ahn, N.; Kwak, K.; Jang, M.S.; Yoon, H.; Lee, B.Y.; Lee, J.K.; Pikhitsa, P.V.; Byun, J.; Choi, M. Trapped Charge-Driven Degradation of Perovskite Solar Cell. Nat. Commun. 2016, 7, 13422–13430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almora, O.; Aranda, C.; Mas-Marzá, E.; Garcia-Belmonte, G. On Mott-Schottky Analysis Interpretation of Capacitance Measurements in Organometal Perovskite Solar Cells. Appl. Phys. Lett. 2016, 109, 173903. [Google Scholar] [CrossRef] [Green Version]
- Galatopoulos, F.; Savva, A.; Papadas, I.T.; Choulis, S.A. The Εffect of Hole Transporting Layer in Charge Accumulation Properties of p-i-n Perovskite Solar Cells. APL Mater. 2017, 5, 076102. [Google Scholar] [CrossRef] [Green Version]






| Device Architecture | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/Al | 0.84 | 15.41 | 75 | 9.68 |
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/γ-Fe2O3/Al | 0.88 | 16.53 | 69.7 | 10.13 |
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/AZO/Al | 0.85 | 15.4 | 74.4 | 9.72 |
| Device Architecture | Rs Fresh (Ω) | Rsh Fresh (kΩ) | Rs Aged (Ω) | Rsh Aged (kΩ) |
|---|---|---|---|---|
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/Al | 9.78 | 37.04 | 2.45 | 11.24 |
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/γ-Fe2O3/Al | 11.8 | 38.23 | 7.18 | 33.66 |
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/AZO/Al | 4.83 | 36.26 | 4.7 | 5.56 |
| Device Architecture | E(Vbi) Drop between HF and LF of Fresh Devices (V) | E(Vbi) Drop between Fresh and Aged Devices (V) |
|---|---|---|
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/Al | 0.73 | 0.39 |
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/γ-Fe2O3/Al | 0.4 | 0.09 |
| ITO/PEDOT:PSS/CH3NH3PbI3/PC(70)BM/AZO/Al | 0.3 | 0.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadas, I.T.; Galatopoulos, F.; Armatas, G.S.; Tessler, N.; Choulis, S.A. Nanoparticulate Metal Oxide Top Electrode Interface Modification Improves the Thermal Stability of Inverted Perovskite Photovoltaics. Nanomaterials 2019, 9, 1616. https://doi.org/10.3390/nano9111616
Papadas IT, Galatopoulos F, Armatas GS, Tessler N, Choulis SA. Nanoparticulate Metal Oxide Top Electrode Interface Modification Improves the Thermal Stability of Inverted Perovskite Photovoltaics. Nanomaterials. 2019; 9(11):1616. https://doi.org/10.3390/nano9111616
Chicago/Turabian StylePapadas, Ioannis T., Fedros Galatopoulos, Gerasimos S. Armatas, Nir Tessler, and Stelios A. Choulis. 2019. "Nanoparticulate Metal Oxide Top Electrode Interface Modification Improves the Thermal Stability of Inverted Perovskite Photovoltaics" Nanomaterials 9, no. 11: 1616. https://doi.org/10.3390/nano9111616