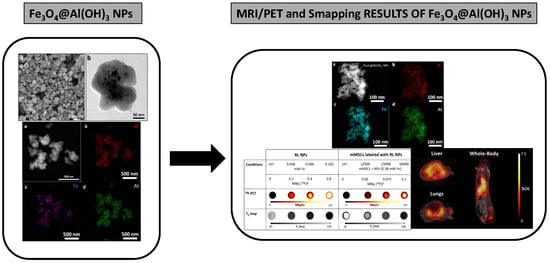
Development of Superparamagnetic Nanoparticles Coated with Polyacrylic Acid and Aluminum Hydroxide as an Efficient Contrast Agent for Multimodal Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Synthesis
2.1.1. Chemicals
2.1.2. Synthesis of Fe3O4 NPs Functionalized with PAA
2.1.3. Synthesis of Functionalized Fe3O4 NPs with PAA Coated Then by a Layer of Al(OH)3
2.2. Physicochemical Characterization
2.2.1. XRD- Structural Characterization
2.2.2. Microscopy Morphological Characterization
2.2.3. Surface Chemistry Characterization
2.2.4. Compositional Characterization
2.2.5. Magnetic Characterization
2.2.6. Preliminary Study of Non-Radioactive 19F Adsorption
2.2.7. Labeling of Fe3O4@Al(OH)3 Nanoparticles with 18F-Sodium Fluoride
2.2.8. [18F]NaF Production
2.2.9. Radiolabeling of Fe3O4@Al(OH)3 NPs
2.2.10. Stability of the Radiolabeling
2.2.11. Dual PET/MRI Scanning
2.3. Biological Characterization
2.3.1. Cell Labelling Using Radiolabelled Fe3O4@Al(OH)3 NPs
2.3.2. Iron Content Measurement in Cell Labelling
2.3.3. In Vitro PET/MR of RL Fe3O4@Al(OH)3 NPs and RL NP-Labelled mMSCs
2.3.4. Image Analysis
MRI
PET
2.3.5. Cell Proliferation and Survival
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
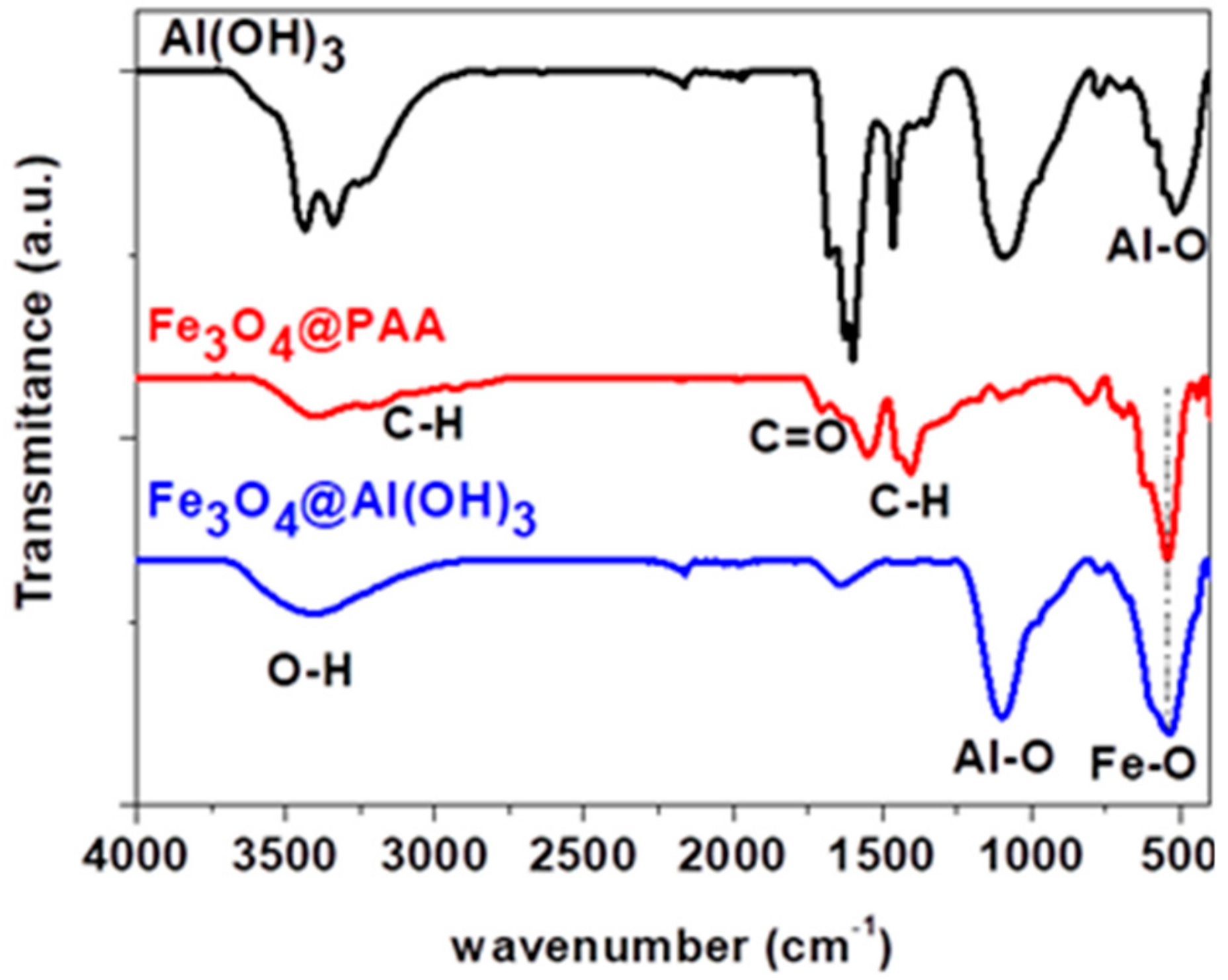
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Spectroscopy
3.3. Magnetic Characterization
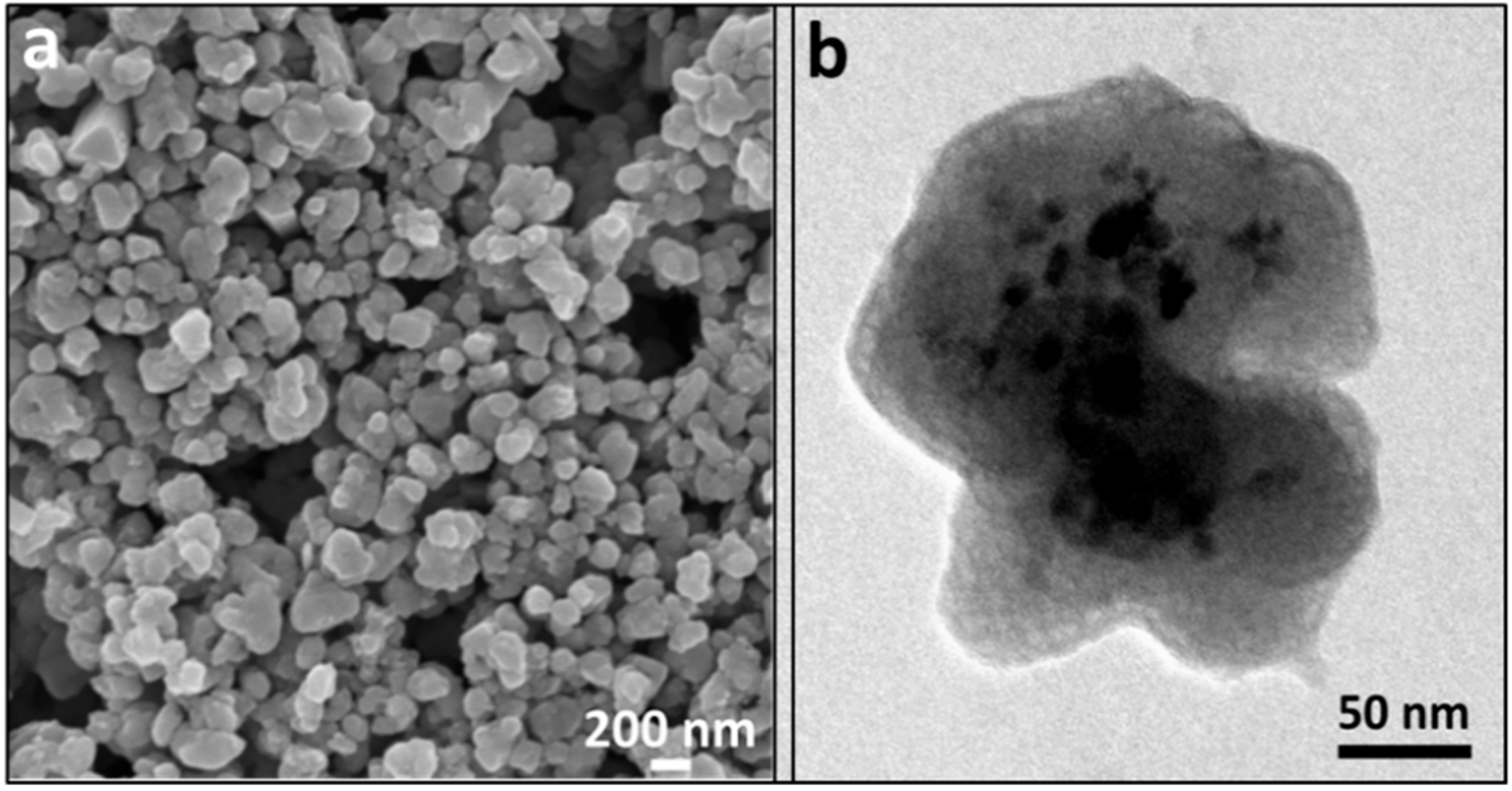
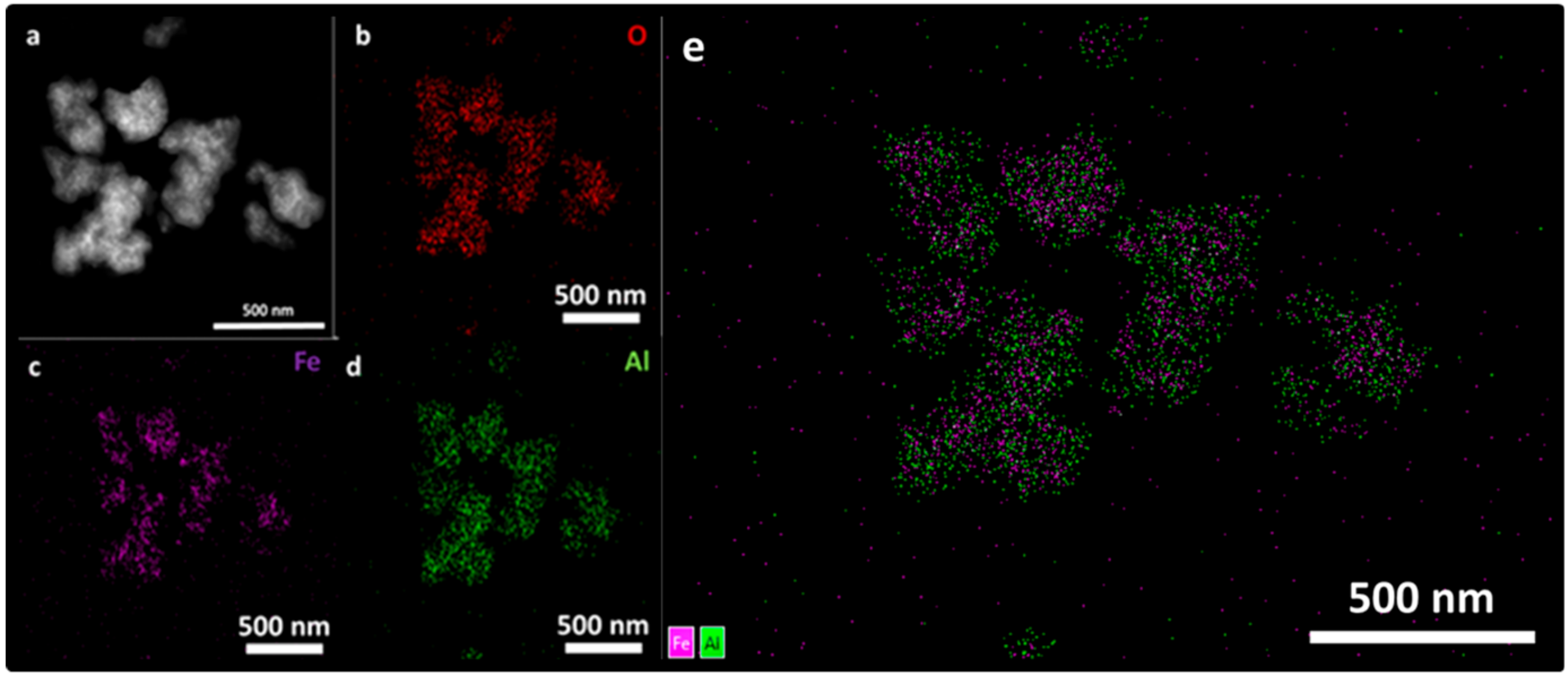
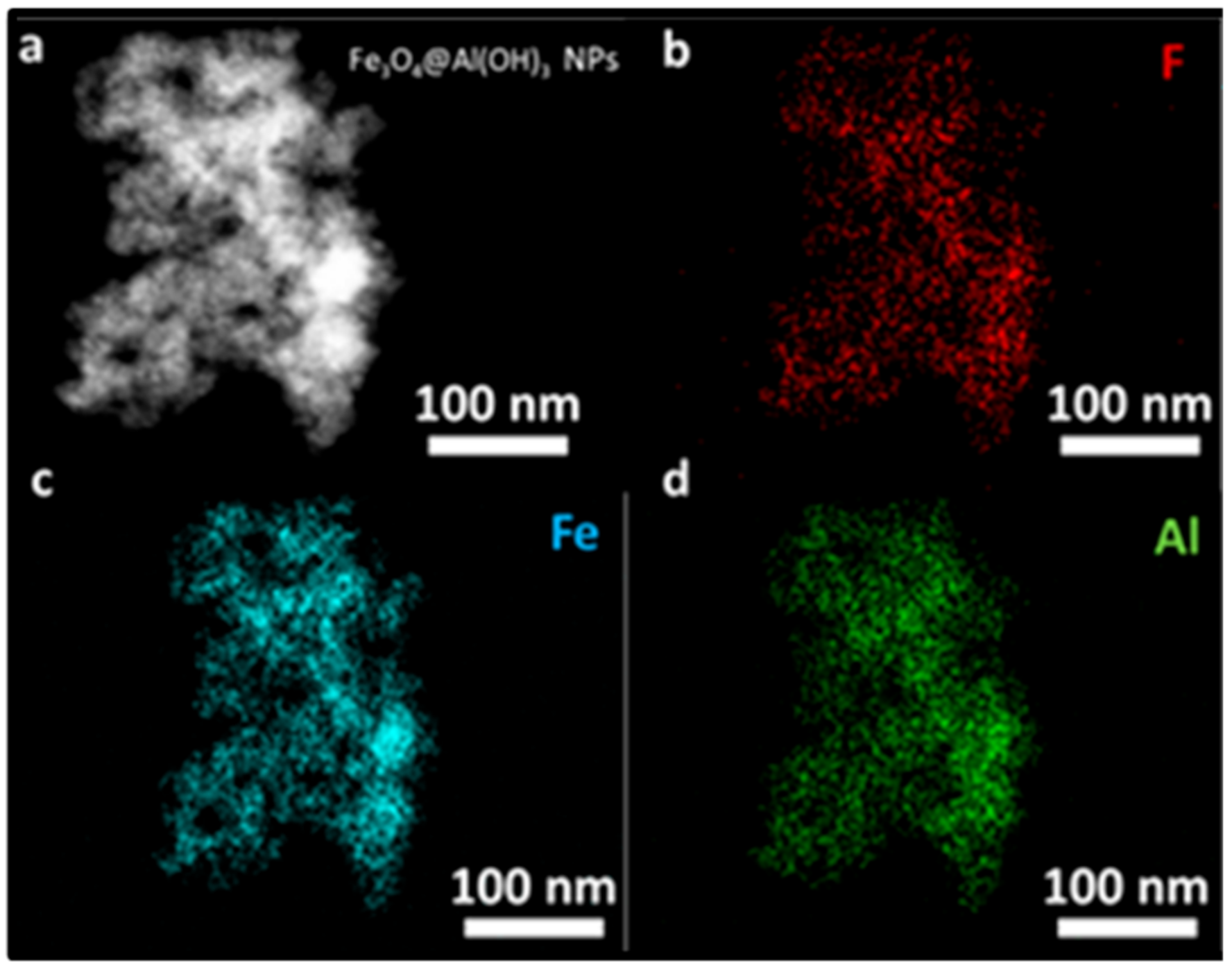
3.4. Transmission and Scanning Electron Microscopy (SEM, TEM, STEM, and EDS Mapping)
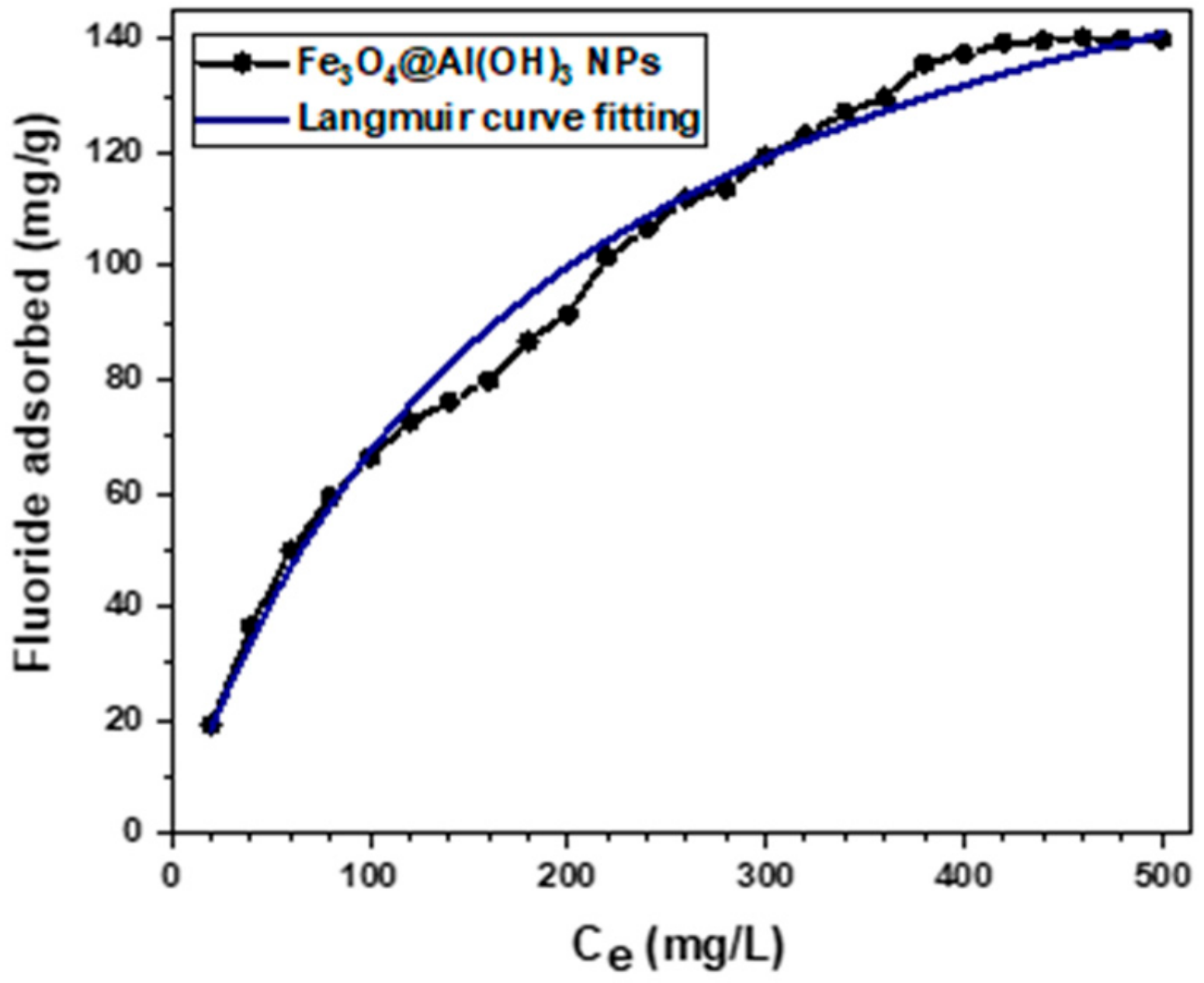
3.5. Preliminary Study of Fluoride Adsorption of Non-Radioactive 19F−
3.6. Time Course and Stability of [18F]NaF complexation to Fe3O4@Al(OH)3 NPs
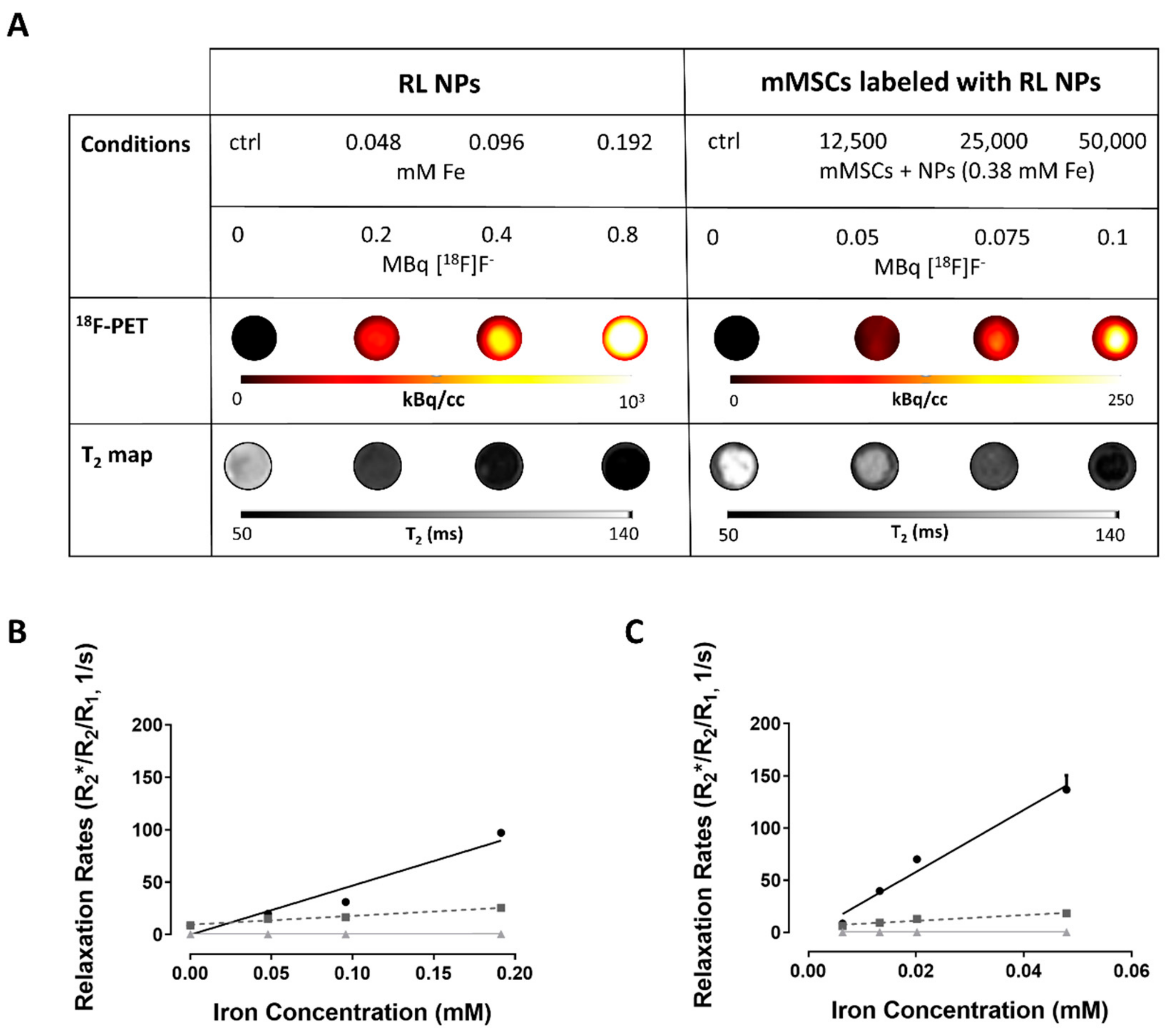
3.7. PET/MRI Visualization of Fe3O4@Al(OH)3 NPs and NP-Loaded mMSC Labelled with [18F]NaF
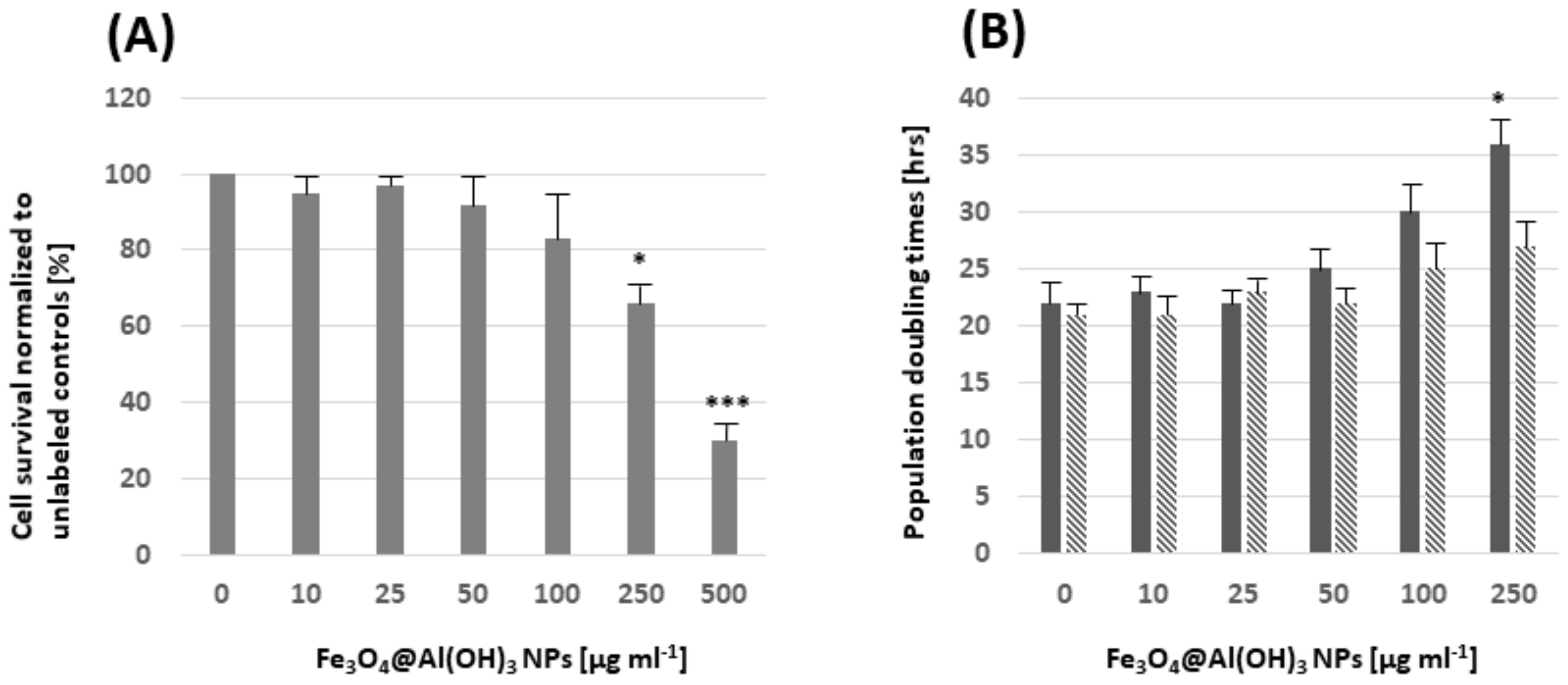
3.8. Assessment of Potentially Toxic Effects by Fe3O4@Al(OH)3 NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | attenuated total reflectance |
| CT | computed tomography |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| ECA | elemental chemical analysis |
| EDS | energy dispersive X-ray spectroscopy |
| EDX | energy dispersive X-ray |
| FAAS | flame atomic absorption spectroscopy |
| FBS | fetal bovine serum |
| Fe3O4@OA | magnetite functionalized with oleic acid |
| FOV | field of view |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GOT | glutamate oxaloacetate transaminase enzyme |
| GPT | glutamate pyruvate transaminase enzyme |
| HMMSN | Hybrid Magnetic Mesoporous Silica Nanorods |
| HAADF | High-Angle Annular Dark Field |
| ICP-OES | inductively coupled plasma optical emission spectroscopy |
| ISE | Ion-Selective electrode |
| iTLC | instant thin layer chromatography |
| (m)MSCs | (mouse) mesenchymal stem cells |
| MNPs | magnetic nanoparticles |
| MRI | Magnetic Resonance Imaging |
| NPs | nanoparticles |
| PBS | Phosphate buffered saline |
| PDT | population doubling times |
| PET | Positron emission tomography |
| RL | radiolabelled |
| Rf | retardation factor |
| Rx | relaxation rate |
| rx | relaxivity |
| SEM | scanning electron microscopy |
| SPECT | single photon emission computed tomography |
| SPM | superparamagnetic |
| STEM | scanning transmission electron microscopy |
| SUV | standardized uptake value |
| TGA | Thermogravimetric analysis |
| TEM | transmission electron microscopy |
| TISAB | III Total Ionic Strength Adjustment Buffer solution |
| VSM | vibrating sample magnetometer |
| XRD | X-ray diffraction. |
References
- Aiello, M.; Cavaliere, C.; Fiorenza, D.; Duggento, A.; Passamonti, L.; Toschi, N. Neuroinflammation in neurodegenerative diseases: Current multi-modal imaging studies and future opportunities for hybrid PET/MRI. Neuroscience 2019, 403, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar]
- Autio, A.; Uotila, S.; Kiugel, M.; Kytö, V.; Liljenbäck, H.; Kudomi, N.; Oikonen, V.; Metsälä, O.; Helin, S.; Knuuti, J.; et al. 68Ga-DOTA chelate, a novel imaging agent for assessment of myocardial perfusion and infarction detection in a rodent model. J. Nucl. Cardiol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Tang, T.; Louie, A. Nanoparticle-based multimodal PET/MRI probes. Nanomedicine 2015, 10, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Eqqvist, J. Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications-Focus on Prostate and Breast Cancer. Int. J. Mol. Sci. 2017, 18, 1102. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhi, D.; Luo, Y.; Zhang, J.; Nan, X.; Zhang, Y.; Zhou, W.; Qiu, B.; Wen, L.; Liang, G. Core/shell Fe3O4/ Gd2O3 nanocubes as T1-T2 dual modal MRI contrast agents. Nanoscale 2016, 8, 12826–12833. [Google Scholar] [CrossRef]
- Kim, D.; Zhang, Y.; Kehr, J.; Klason, T.; Bjelke, B.; Muhammed, M. Characterization and MRI study of surfactant-coated superparamagnetic nanoparticles administered into the rat brain. J. Magn. Magn. Mater. 2001, 225, 256–261. [Google Scholar] [CrossRef]
- Jauregui-Osoro, M.; Williamson, P.; Glaria, A.; Sunassee, K.; Charoenphun, P.; Green, M.; Mullen, G.; Blower, P. Biocompatible inorganic nanoparticles for [18F]–fluoride binding with applications in PET imaging. Dalt. Trans. 2011, 40, 6226–6637. [Google Scholar] [CrossRef]
- Dadfar, M.S.; Roemhild, K.; Drude, N.; von Stillfried, S.; Knuechel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Himmelreich, U.; Hoehn, M. Stem cell labelling for Magnetic Resonance Imaging. Minimally Invasive Minim. Invasive Ther. Allied Technol. 2008, 17, 132–142. [Google Scholar] [CrossRef]
- Liu, S.F.; Laurent, H.; Fattahi, L.V.E.; Muller, R.N. Superparamagnetic nanosystems based on iron oxide nanoparticles for biomedical imaging. Nanomedicine 2011, 6, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, Y.; Vargas, Z.; Rivas, J.; López-Quintela, M.A. Iron Oxide based Nanoparticles for Magnetic Hyperthermia Strategies in Biomedical Applications. Eur. J. Inorg. Chem. 2015, 27, 4495–4509. [Google Scholar] [CrossRef]
- Wang, Y.X. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [PubMed]
- Laurent, S.; Boutry, S.; Mahieu, I.; Elst, L.V.; Muller, R.N. Iron Oxide Based MR Contrast Agents: From Chemistry to Cell Labeling. Curr. Med. Chem. 2009, 16, 4712–4727. [Google Scholar] [CrossRef]
- Sandiford, L.; Phinikaridou, A.; Protti, A.; Meszaros, K.L.; Cui, X.; Yan, Y.; Frodsham, G.; PWilliamson, A.; Gaddum, N.; Botnar, M.R.; et al. Bisphosphonate-anchored PEGylation and radiolabeling of superparamagnetic iron oxide: Long-circulating nanoparticles for in vivo multimodal (T1 MRI-SPECT) imaging. ACS Nano 2012, 7, 500–512. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Aitchison, G.; Curtis, A.S.G. Cell response to dextran-derivatised iron oxide nanoparticles post internalisation. Biomaterials 2004, 25, 5405–5413. [Google Scholar] [CrossRef]
- Himmelreich, U.; Dresselaers, T. Cell labelling and tracking for experimental models using Magnetic Resonance Imaging. Methods 2009, 48, 112–124. [Google Scholar] [CrossRef]
- Argibay, B.; Trekker, J.; Himmelreich, U.; Beiras, A.; Topete, A.; Taboada, P.; Perez-Mato, M.; Iglesias-Rey, R.; Sobrino, T.; Rivas, J.; et al. Easy and efficient cell tagging with block copolymer-based contrast agents for sensitive MRI detection in vivo. Cell Transplant. 2016, 25, 1787–1800. [Google Scholar] [CrossRef]
- Wang, Y.X. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400–13402. [Google Scholar] [CrossRef]
- The NanoTherm® Therapy. Available online: https://www.Magforce.Com/En/Home/Our_Therapy/ (accessed on 20 September 2019).
- Kiani, A.; Esquevin, A.; Lepareur, N.; Bourguet, P.; Jeune, F.L.; Gauvrita, J.Y. Main applications of hybrid PET-MRI contrast agents: A review. Contrast Media Mol. Imaging 2016, 11, 92–98. [Google Scholar] [CrossRef]
- Lee, H.Y.; Li, Z.; Chen, K.; Hsu, A.R.; Xu, C.; Xie, J.; Sun, S.; Chen, X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)–conjugated radiolabelled iron oxide nanoparticles. J. Nucl. Med. 2008, 49, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Belo, S.; Krüger, D.; Yan, Y.; de Rosales, R.T.M.; Jauregui-Osoro, M.; Ye, H.; Su, S.; Mathe, D.; Kovács, N.; et al. Aluminum hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging. Biomaterials 2014, 35, 5840–5846. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Green, A.M.; Blower, J.P.; Zhou, D.; Yan, Y.; Zhang, W.; Djanashvili, K.; Mathe, D.; Veres, K.D.S. Szigeti. Chem. Commun. 2015, 51, 9332–9335. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Chen, W.; Liu, C.; Liu, Y.; Dong, C. Preparation and characterization of γ-AlOOH @CS magnetic nanoparticle as a novel adsorbent for removing fluoride from drinking water. J. Colloid Interface Sci. 2015, 443, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Wang, Y.; Zhao, N.; Yang, W.; You, X. Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res. 2013, 47, 4040–4049. [Google Scholar] [CrossRef] [PubMed]
- Ayoob, S.; Gupta, A.K. Fluoride in Drinking Water: A Review on the Status and Stress Effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Liu, B.; Gao, X.; Xu, W.; Liang, P.; Xu, Y. Adsorption of fluoride from aqueous solution using low-cost bentonite/chitosan beads. Am. J. Anal. Chem. 2013, 4, 48–53. [Google Scholar] [CrossRef]
- Lodhia, J.; Mandarano, G.; Ferris, N.J.; Eu, P.; Cowell, S.F. Development and use of iron oxide nanoparticles (part 1): Synthesis of iron oxide nanoparticles for MRI. Biomed. Imaging Interv. J. 2010, 6, 2–12. [Google Scholar] [CrossRef]
- Xie, L.; Lan, F.; Li, W.; Liu, Z.; Ma, S.; Yang, Q.; Wu, Y.; Gu, Z. Polyacrylic acid brushes grafted from P(St-AA)/Fe3O4 composite microspheres via ARGET-ATRP in aqueous solution for protein immobilization. Colloids Surf. B. Biointerfaces 2014, 123, 413–418. [Google Scholar] [CrossRef]
- Burugapalli, K.; Koul, V.; Dinda, A.K. Effect of composition of interpenetrating polymer network hydrogels based on poly(acrylic acid) and gelatin on tissue response: A quantitative in vivo study. J. Biomed. Mater. Res. 2004, 68, 210–218. [Google Scholar] [CrossRef]
- Massart, R.M. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Roh, H.-S.; Choi, G.-K.; An, J.-S.; Cho, C.-M.; Kim, D.-H.; Park, I.-J.; Noh, T.-H.; Kim, D.-W.; Hong, K.-S. Size-controlled synthesis of monodispersed mesoporous a-Alumina spheres by a template-free forced hydrolysis method. Dalton Trans. 2011, 40, 6901. [Google Scholar] [CrossRef]
- Trekker, J.; Leten, C.; Struys, T.; Lazenka, V.V.; Argibay, B.; Micholt, L.; Lambrichts, I.; Roy, W.V.; Lagae, L.; Himmelreich, U. Sensitive in vivo cell detection using size-optimized superparamagnetic nanoparticles. Biomaterials 2014, 35, 1627–1635. [Google Scholar] [CrossRef]
- Gsell, W.; Molinos, C.; Correcher, C.; Junge, S.; Heidenberg, M.; Himmelreich, U.; Deroose, C.M.; Gonzalez, J.G.A. Characterization of preclinical PET insert for 7T: Beyond NEMA testing. Proc. Intl. Soc. Mag. Reson. Med. 2019, 27, 4358. [Google Scholar]
- Thevenaz, P.; Ruttiman, U.E.; Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998, 7, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Ketkar-Atre, A.; Struys, T.; Soenen, J.S.; Lambrichts, I.; Verfaillie, C.M.; de Cuyper, M.; Himmelreich, U. Variability in contrast agent uptake by different but similar stem cell types. Intl. J. Nanomed. 2013, 8, 4577–4591. [Google Scholar]
- Kimura, S.; Ming, L.C.; Manghnani, H.M.; Nakagiri, N. Physics and Chemistry of Minerals; Springer: Berlin/Heidelberg, Germany, 1986; pp. 238–244. [Google Scholar]
- O’Daniel, H.; Zigan, F.; Rothbauer, R. Zeitschrift fuer Kristallographie, Kristallgeometrie, Kristallphysik. Kristallchemie 1967, 125, 317–331. [Google Scholar]
- Fu, P.; Lu, W.; Lei, W.; Wu, K.; Xu, Y.; Wu, J. Thermal Stability and Microstructure Characterization of MgAl2O4 Nanoparticles Synthesized by Reverse Microemulsion Method. Mater. Res. 2013, 16, 844–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.L.; Bao, J.; Wang, L.; Zhang, F.; Li, Y.D. One-Pot Synthesis and Bioapplication of Amine-Functionalized Magnetite Nanoparticles and Hollow Nanospheres. Chem. Eur. J. 2006, 12, 6341–6347. [Google Scholar] [CrossRef]
- Liao, M.H.; Chen, D.H. Preparation and characterization of a novel magnetic nano-adsorbent. J. Mater. Chem. 2002, 12, 3654–3659. [Google Scholar] [CrossRef]
- Goya, G.F.; Berquo, T.S.; Fonseca, F.C. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, A.; Pius, A. Removal of fluoride from drinking water using aluminum hydroxide coated activated carbon prepared from bark of Morinda tinctorial. Appl. Water Sci. 2017, 7, 2653–2665. [Google Scholar] [CrossRef] [Green Version]
- Rosenqvist, J.; Persson, P.; Sjöberg, S. Protonation and charging of nanosized gibbsite (α-Al(OH)3) particles in aqueous suspension. Langmuir 2002, 18, 4598–4604. [Google Scholar] [CrossRef]
- Zhao, X.; Wanga, J.; Wub, F.; Wanga, T.; Caia, Y.; Shia, Y.; Jianga, G. Removal of fluoride from aqueous media by Fe3O4@Al(OH)3 magnetic nanoparticles. J. Hazard. Mater. 2010, 173, 102–109. [Google Scholar] [CrossRef]
- Bitton, G. Formula Handbook for Environmental Engineers and Scientist; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Kumar, V.K.; Sivanesan, S. Selection of optimum sorption kinetics: Comparison of linear and non-linear method. J. Hazard. Mater. 2006, 134, 277–279. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221. [Google Scholar] [CrossRef] [Green Version]
- Klug, G.; Kampf, T.; Bloemer, S.; Bremicker, J.; Ziener, C.H.; Andrea, H.; Bureck, U.G.; Rommel, E.; Nöth, U.; Schenk, W.A.; et al. Intracellular and extracellular T1 an T2 relaxivities of magneto-optical nanoparticles at experimental high fields. Magn. Reson. Med. 2010, 64, 1607–1615. [Google Scholar] [CrossRef]
- Richard, S.; Boucher, M.; Lalatonne, Y.; Mériaux, S.; Motte, M. Iron oxide nanoparticle surface decorated with cRGD peptides for magnetic resonance imaging of brain tumors. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1515–1520. [Google Scholar] [CrossRef]
- Aarntzen, E.H.J.G.; Figdor, C.G.; Adema, G.J.; Punt, C.J.A.; de Vries, I.J.M. Dendritic cell vaccination and immune monitoring. Cancer Immunol. Immunother 2008, 57, 1559–1568. [Google Scholar] [CrossRef] [Green Version]
- Jirak, D.J.K.; Berkova, Z.; Herynek, V.; Lodererova, A.; Girman, P.; Habart, D.; Hajek, M.; Saudek, F. Detection of pancreatic islet allograft impairment in advance of functional failure using magnetic resonance imaging. Transpl. Int. 2012, 25, 250–260. [Google Scholar]
- Malosio, M.L.; Esposito, A.; Brigatti, C.; Palmisano, A.; Piemonti, L.; Nano, R.; Maffi, P.; de Cobelli, A.F.; Maschio, D.; Secchi, A. MR imaging monitoring of iron labelled pancreatic islets in a small series of patients: Islets fate in successful, unsuccessful and auto-transplantation. Cell Transpl. 2015, 24, 2285–2296. [Google Scholar] [CrossRef]
- De Chickera, S.; Willert, C.; Mallet, C.; Foley, R.; Foster, P.; Dekaban, G.A. Cellular MRI as a suitable, sensitive non-invasive modality for correlating in vivo migratory efficiencies of different dendritic cell populations with subsequent immunological outcomes. Int. Immunol. 2011, 24, 29–41. [Google Scholar]
- De Temmerman, M.-L.; Soenen, S.J.; Symens, N.; Lucas, B.; Vandenbroucke, R.E.; Libert, C.; Demeester, J.; de Smedt, S.C.; Himmelreich, U.; Rejman, J.R. Magnetic layer-by-layer coated particles for efficient magnetic resonance imaging of dendritic cells and mesenchymal stem cells. Nanomedicine 2014, 9, 1363–1376. [Google Scholar] [CrossRef]
- Argibay, B.; Trekker, J.; Himmelreich, U.; Beiras, A.; Topete, A.; Taboada, P.; Pérez-Mato, M.; Vieites-Prado, A.; Iglesias-Rey, R.; Rivas, J.; et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci. Rep. 2017, 7, 40758. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297–304. [Google Scholar] [CrossRef] [Green Version]
- McBride, W.J.; Sharkey, R.M.; Goldenberg, D.M. Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res. 2013, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cleeren, F.; Lecina, J.; Billaud, E.M.F.; Ahamed, M.; Verbruggen, A.; Bormans, G.M. New Chelators for Low Temperature Al18 F-Labelling of Biomolecules. Bioconjug. Chem. 2016, 27, 790–798. [Google Scholar] [CrossRef] [Green Version]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem Cell Transplantation: The Lung Barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef] [Green Version]
- Kiessling, F.K. Noninvasive Cell Tracking. In Molecular Imaging II; Springer: Berlin/Heidelberg, Germany, 2008; pp. 305–321. [Google Scholar]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- De Jong, H.W.A.M.; Perk, L.; Visser, G.W.M.; Boellaard, R.; van Dongen, G.A.M.S.; Lammertsma, A.A. High Resolution PET Imaging Characteristics of 68Ga, 124I and 89Zr Compared to 18F. In Proceedings of the IEEE Nuclear Science Symposium Conference Record, 2005, Fajardo, Puerto Rico, 23–29 October 2005; Volume 3, pp. 1624–1627. [Google Scholar]
- Lahooti, A.; Sarkar, S.; Laurent, S.; Shanehsazzadeh, S. Dual nano-sized contrast agents in PET/MRI: A systematic review. Contrast Media Mol. Imaging. 2016, 11, 428–447. [Google Scholar] [CrossRef] [Green Version]
- Mannheim, J.G.; Schmid, A.M.; Schwenck, J.; Katiyar, P.; Herfert, K.; Pichler, B.J.; Disselhorst, J.A. PET/MRI Hybrid Systems. Semin. Nucl. Med. 2018, 48, 332–347. [Google Scholar] [CrossRef]



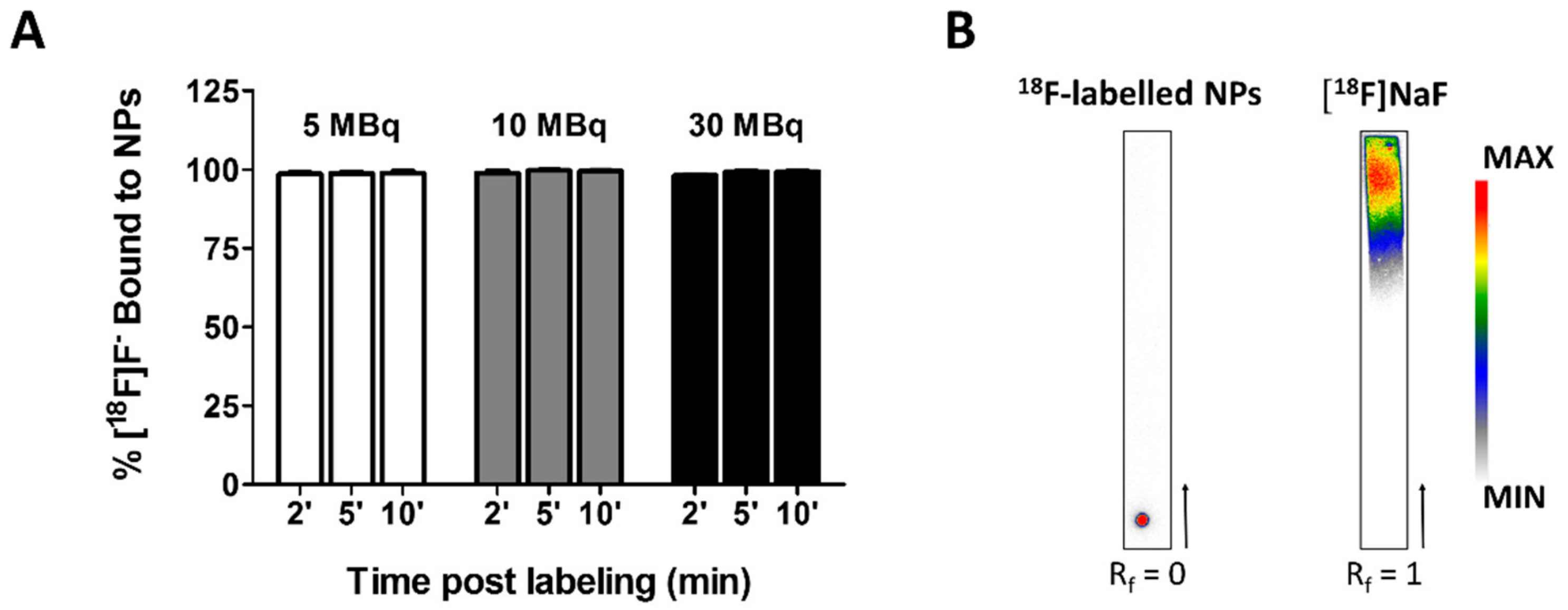
| [18F]F− Bound to NPs after Storage at 37 °C for | ||||||||
|---|---|---|---|---|---|---|---|---|
| 30 min | 1 h | 2 h | 4 h | |||||
| % | SD | % | SD | % | SD | % | SD | |
| Milli-Q water | 65.6 | 17.2 | 85.6 | 5.2 | 77.9 | 6.3 | 86.7 | 8.3 |
| Saline | 69.5 | 2.6 | 66.8 | 12.9 | 78.5 | 12.7 | 76.9 | 12.4 |
| PBS | 57.4 | 5.6 | 53.8 | 12.7 | 38.5 | 2.1 | 40.1 | 5.0 |
| TrypLE | 53.1 | 5.7 | 61.1 | 19.6 | 38.2 | 5.1 | 36.9 | 3.4 |
| mMSC medium | 40.4 | 7.9 | 46.9 | 7.4 | 38.3 | 8.9 | 28.9 | 1.7 |
| 50% medium/ 50% FBS | 36.8 | 3.3 | 58.5 | 14.8 | 40.3 | 3.2 | 40.4 | 5.2 |
| FBS | 35.7 | 1.8 | 46.5 | 8.1 | 32.5 | 3.3 | 34.4 | 10.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Gómez, M.A.; Belderbos, S.; Yañez-Vilar, S.; Piñeiro, Y.; Cleeren, F.; Bormans, G.; Deroose, C.M.; Gsell, W.; Himmelreich, U.; Rivas, J. Development of Superparamagnetic Nanoparticles Coated with Polyacrylic Acid and Aluminum Hydroxide as an Efficient Contrast Agent for Multimodal Imaging. Nanomaterials 2019, 9, 1626. https://doi.org/10.3390/nano9111626
González-Gómez MA, Belderbos S, Yañez-Vilar S, Piñeiro Y, Cleeren F, Bormans G, Deroose CM, Gsell W, Himmelreich U, Rivas J. Development of Superparamagnetic Nanoparticles Coated with Polyacrylic Acid and Aluminum Hydroxide as an Efficient Contrast Agent for Multimodal Imaging. Nanomaterials. 2019; 9(11):1626. https://doi.org/10.3390/nano9111626
Chicago/Turabian StyleGonzález-Gómez, Manuel Antonio, Sarah Belderbos, Susana Yañez-Vilar, Yolanda Piñeiro, Frederik Cleeren, Guy Bormans, Christophe M. Deroose, Willy Gsell, Uwe Himmelreich, and José Rivas. 2019. "Development of Superparamagnetic Nanoparticles Coated with Polyacrylic Acid and Aluminum Hydroxide as an Efficient Contrast Agent for Multimodal Imaging" Nanomaterials 9, no. 11: 1626. https://doi.org/10.3390/nano9111626
APA StyleGonzález-Gómez, M. A., Belderbos, S., Yañez-Vilar, S., Piñeiro, Y., Cleeren, F., Bormans, G., Deroose, C. M., Gsell, W., Himmelreich, U., & Rivas, J. (2019). Development of Superparamagnetic Nanoparticles Coated with Polyacrylic Acid and Aluminum Hydroxide as an Efficient Contrast Agent for Multimodal Imaging. Nanomaterials, 9(11), 1626. https://doi.org/10.3390/nano9111626