V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Electrochemical Measurements
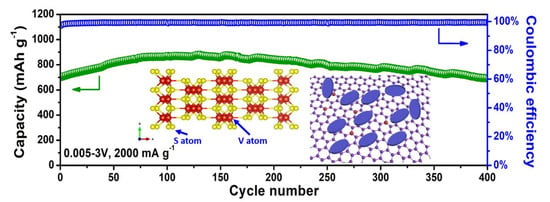
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Liu, J.; Chao, D.; Mai, L. Vanadate-Based Materials for Li-Ion Batteries: The Search for Anodes for Practical Applications. Adv. Energy Mater. 2019, 9, 1803324. [Google Scholar] [CrossRef]
- Jiang, L.; Qu, Y.; Ren, Z.; Yu, P.; Zhao, D.; Zhou, W.; Wang, L.; Fu, H. In Situ Carbon-Coated Yolk–Shell V2O3 Microspheres for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zheng, C.; Xi, J.; Fei, H.; Wei, M. Composites of V2O3-ordered mesoporous carbon as anode materials for lithium-ion batteries. Carbon 2013, 62, 382–388. [Google Scholar] [CrossRef]
- Pan, J.; Zhong, L.; Li, M.; Luo, Y.; Li, G. Microwave-Assisted Solvothermal Synthesis of VO2 Hollow Spheres and Their Conversion into V2O5 Hollow Spheres with Improved Lithium Storage Capability. Chem. Eur. J. 2016, 22, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wen, Y.; Wu, C.; van Aken, P.A.; Maier, J.; Yu, Y. 3D V6O13 Nanotextiles Assembled from Interconnected Nanogrooves as Cathode Materials for High-Energy Lithium Ion Batteries. Nano Lett. 2015, 15, 1388–1394. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, W.; Zhan, L.; Wang, Y.; Zhu, Y. V3S4 nanoparticles anchored on three-dimensional porous graphene gel for superior lithium storage. Electrochim. Acta 2018, 261, 35–41. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, G.; Lin, Y.; Wang, Y.; Ou, X.; Zheng, F.; Yang, C.; Wang, J.; Liu, M. Enhancing Sodium Ion Battery Performance by Strongly Binding Nanostructured Sb2S3 on Sulfur-Doped Graphene Sheets. ACS Nano 2016, 10, 10953–10959. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.; Zhang, Y.; Liu, H.; Liu, H.; Wu, H.; Dou, S. Graphene-scroll-sheathed α-MnS coaxial nanocables embedded in N, S Co-doped graphene foam as 3D hierarchically ordered electrodes for enhanced lithium storage. Energy Storage Mater. 2019, 16, 46–55. [Google Scholar] [CrossRef]
- Yu, D.; Pang, Q.; Gao, Y.; Wei, Y.; Wang, C.; Chen, G.; Du, F. Hierarchical Flower-like VS2 Nanosheets-A High Rate-Capacity and Stable Anode Material for Sodium-ion Battery. Energy Storage Mater. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Yang, J.; Tian, J.; Xu, H.; Yang, J.; Fan, W. Conductive Polymer-Coated VS4 Submicrospheres as Advanced Electrode Materials in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 18797–18805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, J.; Xu, H.; Yang, J.; Qian, Y. VS4 Nanoparticles Rooted by a-C Coated MWCNTs as an Advanced Anode Material in Lithium Ion Batteries. Energy Storage Mater. 2017, 6, 149–156. [Google Scholar] [CrossRef]
- Yang, G.; Wang, H.; Zhang, B.; Foo, S.; Ma, M.; Cao, X.; Liu, J.; Ni, S.; Srinivasan, M.; Huang, Y. Superior Li-ion storage of VS4 nanowires anchored on reduced graphene. Nanoscale 2019, 11, 9556–9562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Y.; Wang, J.; Zhu, X. Sodium storage performance and mechanism of rGO-wrapped nanorod vanadium sulfide as an anode material for sodium ion batteries. Solid State Ionics 2018, 327, 129–135. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Y.; Wu, F.; Moudrakovski, I.; Aken, P.A.; Maier, J.; Yu, Y. Hierarchical Metal Sulfide/Carbon Spheres: A Generalized Synthesis and High Sodium-Storage Performance. Angew. Chem. Int. Ed. 2019, 58, 7238–7243. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ou, X.; Xiong, X.; Zheng, F.; Hu, R.; Chen, Y.; Liu, M.; Huang, K. V5S8-graphite hybrid nanosheets as a high rate-capacity and stable anode material for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 107–113. [Google Scholar] [CrossRef]
- Ou, X.; Liang, X.; Zheng, F.; Pan, Q.; Zhou, J.; Xiong, X.; Yang, C.; Hu, R.; Liu, M. Exfoliated V5S8/graphite nanosheet with excellent electrochemical performance for enhanced lithium storage. Chem. Eng. J. 2017, 320, 485–493. [Google Scholar] [CrossRef]
- Rout, C.S.; Kim, B.; Xu, X.; Yang, J.; Jeong, H.Y.; Odkhuu, D.; Park, N.; Cho, J.; Shin, H.S. Synthesis and characterization of patronite form of vanadium sulfide on graphitic layer. J. Am. Chem. Soc. 2013, 135, 8720–8725. [Google Scholar] [CrossRef]
- Wu, N.; Tian, W.; Shen, J.; Qiao, X.; Sun, T.; Wu, H.; Zhao, J.; Liu, X.; Zhang, Y. Facile fabrication of a jarosite ultrathin KFe3(SO4)2(OH)6@rGO nanosheet hybrid composite with pseudocapacitive contribution as a robust anode for lithium-ion batteries. Inorg. Chem. Front. 2019, 6, 192–198. [Google Scholar] [CrossRef]
- Wu, N.; Shen, J.; Sun, L.; Yuan, M.; Shao, Y.; Ma, J.; Liu, G.; Guo, D.; Liu, X.; He, Y. Hierarchical N-doped graphene coated 1D cobalt oxide microrods for robust and fast lithium storage at elevated temperature. Electrochim. Acta 2019, 310, 70–77. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, S.; Jeon, K.M.; Piao, Y.; Kang, Y.C. Mesoporous reduced graphene oxide/WSe2 composite particles for efficient sodium-ion batteries and hydrogen evolution reactions. Appl. Surf. Sci. 2018, 459, 309–317. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.Y.; Lee, J.K.; Kang, Y.C. Iron Telluride-Decorated Reduced Graphene Oxide Hybrid Microspheres as Anode Materials with Improved Na-Ion Storage Properties. ACS Appl. Mater. Interfaces 2016, 8, 21343–21349. [Google Scholar] [CrossRef] [PubMed]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V 2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Liu, G.; Cui, J.; Luo, R.; Huang, X.; Wu, N.; Jin, X.; Chen, H.; Tang, S.; et al. 2D MoS2 grown on biomass-based hollow carbon fibers for energy storage. Appl. Surf. Sci. 2019, 469, 854–863. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Yang, W.; Liang, D.; Liu, L.; Liang, S.; Yang, C.; Fang, Z.; Dong, Q.; Deng, C. N/S-Co-Doped Porous Carbon Sheets Derived from Bagasse as High-Performance Anode Materials for Sodium-Ion Batteries. Nanomaterials 2019, 9, 1203. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Tian, Y. Earthworm-like N, S-Doped carbon tube-encapsulated Co9S8 nanocomposites derived from nanoscaled metal–organic frameworks for highly efficient bifunctional oxygen catalysis. J. Mater. Chem. A 2018, 6, 5935–5943. [Google Scholar] [CrossRef]
- Lee, W.J.; Lim, J.; Kim, S.O. Nitrogen Dopants in Carbon Nanomaterials: Defects or a New Opportunity? Small Methods 2017, 1, 1600014. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Wu, J.; Zhang, L.; Wei, M. Facile synthesis of VN hollow spheres as an anode for lithium-ion battery. J. Electroanal. Chem. 2019, 848, 113360. [Google Scholar] [CrossRef]
- Balamurugan, J.; Karthikeyan, G.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Facile synthesis of vanadium nitride/nitrogen-doped graphene composite as stable high performance anode materials for supercapacitors. J. Power Sour. 2016, 308, 149–157. [Google Scholar] [CrossRef]
- Li, Z.; Yin, Q.; Hu, W.; Zhang, J.; Guo, J.; Chen, J.; Sun, T.; Du, C.; Shu, J.; Yu, L.; et al. Tin/tin antimonide alloy nanoparticles embedded in electrospun porous carbon fibers as anode materials for lithium-ion batteries. J. Mater. Sci. 2019, 54, 9025–9033. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, S.; Xi, B.; Feng, Z.; Sun, D.; Ma, X.; Zhang, J.; Feng, J.; Xiong, S. Unusual Formation of CoO@C “Dandelions” Derived from 2D Kagóme MOLs for Efficient Lithium Storage. Adv. Energy Mater. 2018, 8, 1703242. [Google Scholar] [CrossRef]
- Zeng, H.; Xing, B.; Chen, L.; Yi, G.; Huang, G.; Yuan, R.; Zhang, C.; Cao, Y.; Chen, Z. Nitrogen-Doped Porous Co3O4/Graphene Nanocomposite for Advanced Lithium-Ion Batteries. Nanomaterial 2019, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Britto, S.; Leskes, M.; Hua, X.; Hebert, C.A.; Shin, H.S.; Clarke, S.; Borkiewicz, O.; Chapman, K.W.; Seshadri, R.; Cho, J.; et al. Multiple Redox Modes in the Reversible Lithiation of High-Capacity, Peierls-Distorted Vanadium Sulfide. J. Am. Chem. Soc. 2015, 137, 8499–8508. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3-x. Nat. Mater. 2016, 16, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.; Tolbert, S.H.; Abruna A, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wei, Z.; Cui, C.; Liu, Q.; Wang, C.; Ma, J. Oxygen-deficient anatase TiO2@C nanospindles with pseudocapacitive contribution for enhancing lithium storage. J. Mater. Chem. A 2018, 6, 4013–4022. [Google Scholar] [CrossRef]
- Wu, N.; Wu, H.; Qiao, X.; Shen, J.; Liu, X.; Liu, G.; Sun, T.; Hou, H.; Zhang, Y.; Ji, X. Anatase inverse opal TiO2-x@N-doped C induced the dominant pseudocapacitive effect for durable and fast lithium/sodium storage. Electrochim. Acta 2019, 299, 540–548. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, J.K.; Kang, Y.C. Graphitic Carbon-Coated FeSe2 Hollow Nanosphere-Decorated Reduced Graphene Oxide Hybrid Nanofibers as an Efficient Anode Material for Sodium Ion Batteries. Sci. Rep. 2016, 6, 23699. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.Y.; Kang, Y.C. First Introduction of NiSe2 to Anode Material for Sodium-Ion Batteries: A Hybrid of Graphene-Wrapped NiSe2/C Porous Nanofiber. Sci. Rep. 2016, 6, 23338. [Google Scholar] [CrossRef]
- Wu, N.; Wu, H.; Kim, J.; Liu, X.; Zhang, Y. Restoration of Degraded Nickel-Rich Cathode Materials for Long-Life Lithium-Ion Batteries. Chemelectrochem 2018, 5, 78–83. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Zhao, Y.; Wang, Y.; Wang, Z.; Zhang, W.; Ren, F. Facile Synthesis of Two-Dimensional Porous MgCo2O4 Nanosheets as Anode for Lithium-Ion Batteries. Appl. Sci. 2018, 8, 22. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Miao, D.; Zhou, X.; Zhang, L.; Liu, G.; Guo, D.; Liu, X. V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage. Nanomaterials 2019, 9, 1638. https://doi.org/10.3390/nano9111638
Wu N, Miao D, Zhou X, Zhang L, Liu G, Guo D, Liu X. V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage. Nanomaterials. 2019; 9(11):1638. https://doi.org/10.3390/nano9111638
Chicago/Turabian StyleWu, Naiteng, Di Miao, Xinliang Zhou, Lilei Zhang, Guilong Liu, Donglei Guo, and Xianming Liu. 2019. "V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage" Nanomaterials 9, no. 11: 1638. https://doi.org/10.3390/nano9111638
APA StyleWu, N., Miao, D., Zhou, X., Zhang, L., Liu, G., Guo, D., & Liu, X. (2019). V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage. Nanomaterials, 9(11), 1638. https://doi.org/10.3390/nano9111638