Selective Recovery of Europium and Yttrium Ions with Cyanex 272-Polyacrylonitrile Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of PAN Nanofibers by Electrospinning
2.3. Impregnation of Electrospun PAN Nanofibers with Cyanex 272
2.4. Characterization of PAN and PAN-272 Nanofibers
2.5. Adsorption Experiments in Batch Mode
2.5.1. Effect of the Contact Time in the Adsorption Process
2.5.2. Maximum Loading Capacity of the Adsorbent System
2.5.3. Selectivity Adsorption of Eu(III) and Y(III) in Presence of Interfering Heavy Metal Ions
2.5.4. Selectivity towards Most Common Interfering Anions
2.5.5. Adsorption–Desorption Experiments
2.6. Adsorption Experiments in Continuous Mode
3. Results and Discussion
3.1. Characterization of Electrospun PAN Nanofibers
3.1.1. Scanning Electron Microscopy (SEM)
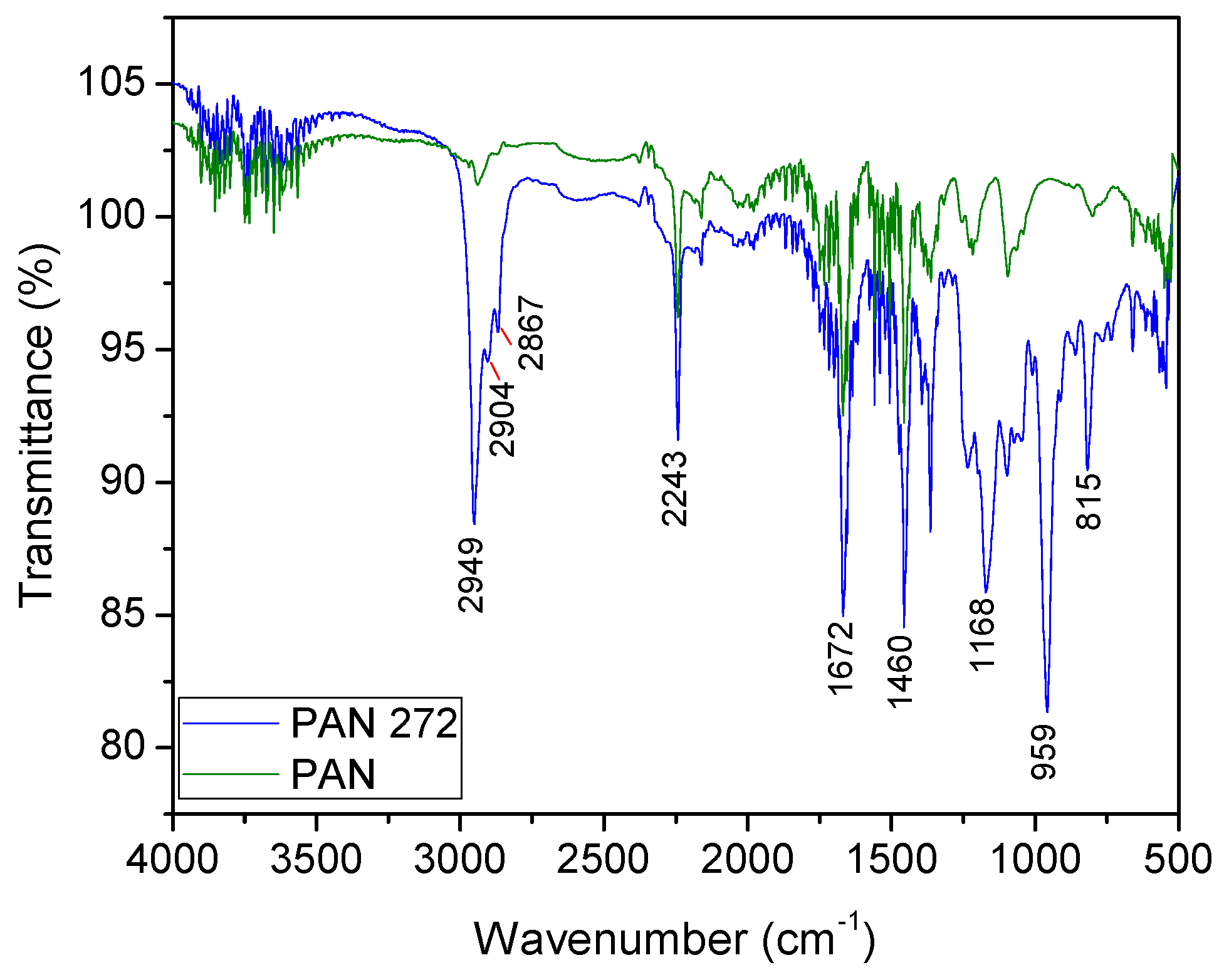
3.1.2. Fourier Transformed Infrared Coupled ATR (ATR-FTIR)
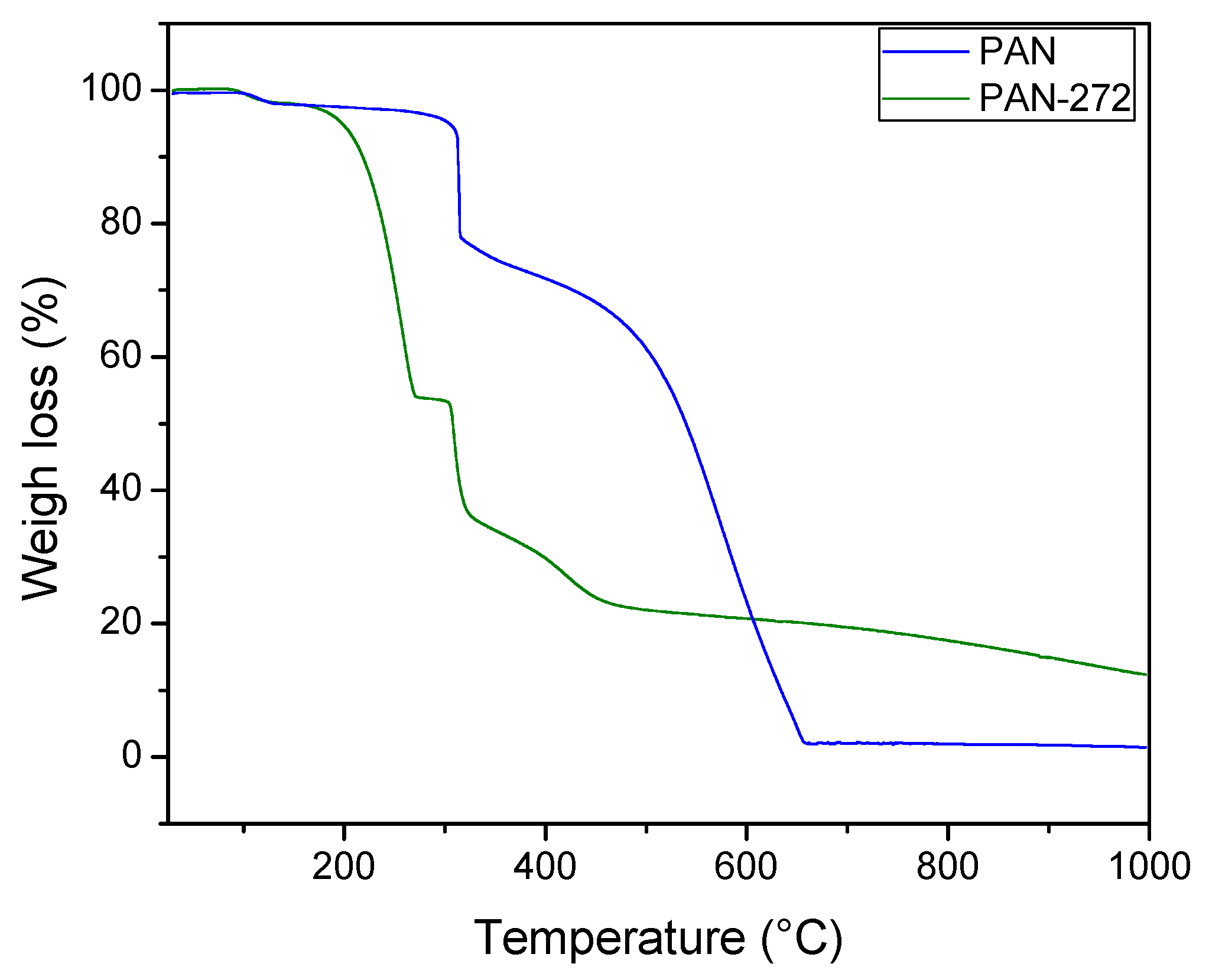
3.1.3. Thermogravimetrical Analysis
3.2. Adsorption Properties of PAN-272 Nanofibers in Batch Mode
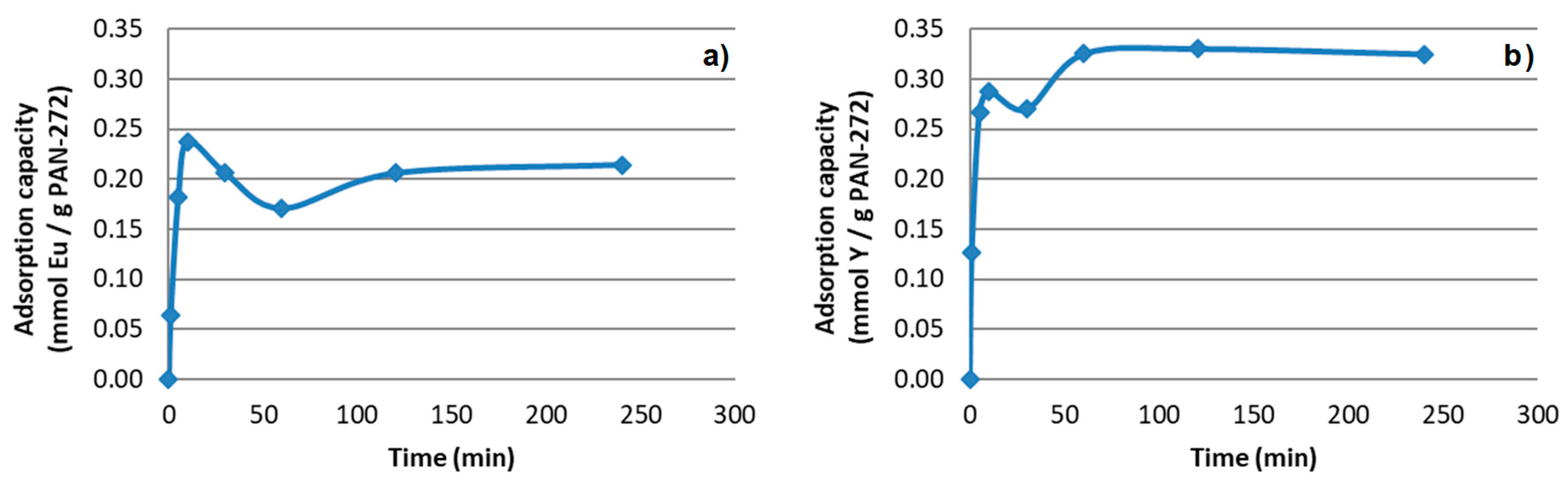
3.2.1. Effect of the Contact Time on the Adsorption Process
3.2.2. Maximum Loading Capacity of the Adsorbent System
3.2.3. Adsorption Isotherms
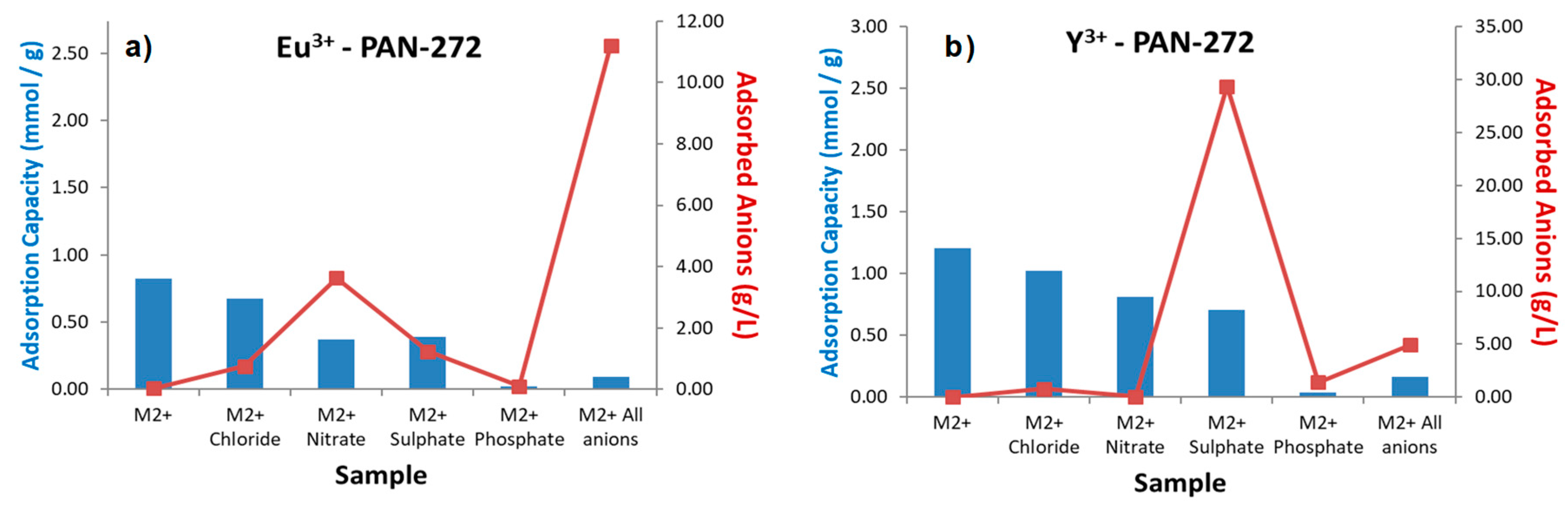
3.2.4. Selectivity towards Most Common Interfering Ions
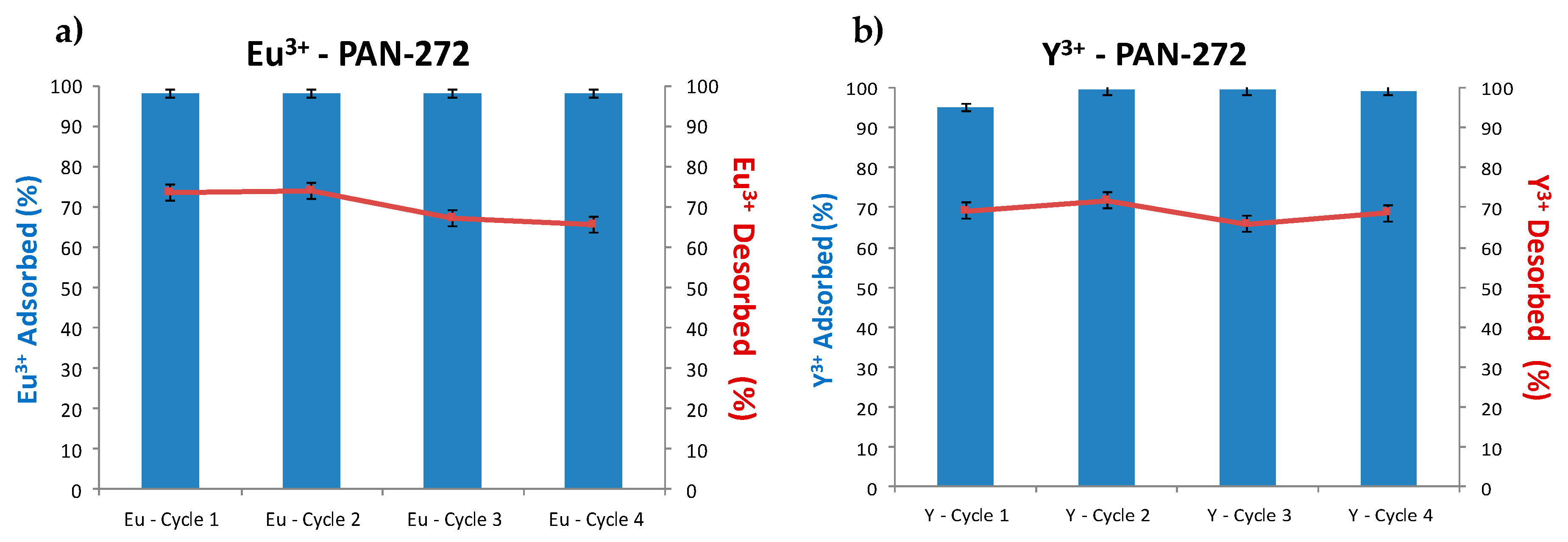
3.2.5. Adsorption–Desorption Cycles and Stripping Experiments
3.3. Adsorption Properties of PAN-272 Nanofibers in Continuous Mode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hofmann, M.; Hofmann, H.; Hagelüken, C.; Hool, A. Critical raw materials: A perspective from the materials science community. Sustain. Mater. Technol. 2018, 17, 1–10. [Google Scholar]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Song, Y.; Yin, D.; Hammouda, S.B.; Chen, L.; Kalliola, S.; Tang, J.; Tam, K.C.; Sillanpää, M. Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study. Green Chem. 2017, 19, 4816–4828. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 561, 1–22. [Google Scholar] [CrossRef]
- Fernandez, V. Rare-earth elements market: A historical and financial perspective. Resour. Policy 2017, 53, 26–45. [Google Scholar] [CrossRef]
- Lee, C.H.; Liao, C.H.; Popuri, S.R.; Hung, C.E. Integrated process development for the recovery of Europium and Yttrium from waste fluorescent powder. J. Mater. Cycles Waste Manag. 2017, 19, 1235–1243. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Karshigina, Z.B.; Bochevskaya, Y.G.; Akcil, A.; Sargelova, E.A.; Kvyatkovskaya, M.N.; Silachyov, I.Y. Recovery of rare earth metals as critical raw materials from phosphorus slag of long-term storage. Hydrometallurgy 2017, 173, 271–282. [Google Scholar] [CrossRef]
- Maes, S.; Zhuang, W.Q.; Rabaey, K.; Alvarez-Cohen, L.; Hennebel, T. Concomitant Leaching and Electrochemical Extraction of Rare Earth Elements from Monazite. Environ. Sci. Technol. 2017, 51, 1654–1661. [Google Scholar] [CrossRef]
- Liao, C.; Nie, H.; Jiao, Y.; Liang, Y.; Yang, S. Study on the diffusion kinetics of adsorption of heavy rare earth with Cyanex272-P507 impregnated resin. J. Rare Earths 2010, 18, 120–124. [Google Scholar] [CrossRef]
- Ngomsik, A.F.; Bee, A.; Talbot, D.; Cote, G. Magnetic solid-liquid extraction of Eu(III), La(III), Ni(II) and Co(II) with maghemite nanoparticles. Sep. Purif. Technol. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Swain, B.; Otu, E.O. Competitive extraction of lanthanides by solvent extraction using Cyanex 272: Analysis, classification and mechanism. Sep. Purif. Technol. 2011, 83, 82–90. [Google Scholar] [CrossRef]
- Reddy, B.R.; Kumar, J.R. Rare Earths Extraction, Separation, and Recovery from Phosphoric Acid Media. Solvent Extr. Ion Exch. 2016, 34, 226–240. [Google Scholar] [CrossRef]
- Kashi, E.; Habibpour, R.; Gorzin, H.; Maleki, A. Solvent extraction and separation of light rare earth elements (La, Pr and Nd) in the presence of lactic acid as a complexing agent by Cyanex 272 in kerosene and the effect of citric acid, acetic acid and Titriplex III as auxiliary agents. J. Rare Earths 2018, 36, 317–323. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. An overview of rare-earth recovery by ion-exchange leaching from ion-adsorption clays of various origins. Mineral. Mag. 2016, 80, 63–76. [Google Scholar] [CrossRef]
- Binnemans, K.; Dupont, D. Process for Recovery of Yttrium and Europium from Lamp Phospor Waste. WO Patent 2016065433 A1, 6 May 2016. [Google Scholar]
- Yang, F.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Selective extraction and recovery of rare earth metals from phosphor powders in waste fluorescent lamps using an ionic liquid system. J. Hazard. Mater. 2013, 254, 79–88. [Google Scholar] [CrossRef]
- Sui, N.; Huang, K.; Zhang, C.; Wang, N.; Wang, F.; Liu, H. Light, middle, and heavy rare-earth group separation: A new approach via a liquid-liquid-liquid three-phase system. Ind. Eng. Chem. Res. 2013, 52, 5997–6008. [Google Scholar] [CrossRef]
- İnan, S.; Tel, H.; Sert, Ş.; Çetinkaya, B.; Sengül, S.; Özkan, B.; Altaş, Y. Extraction and separation studies of rare earth elements using Cyanex 272 impregnated Amberlite XAD-7 resin. Hydrometallurgy 2018, 181, 156–163. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef]
- Martín, D.M.; Ahmed, M.M.; Rodríguez, M.; García, M.A.; Faccini, M. Aminated Polyethylene Terephthalate (PET) nanofibers for the selective removal of Pb(II) from polluted water. Materials 2017, 10, 1352. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Xu, Y.; Liao, S.; Yang, D.; Lo Hsieh, Y.; Wei, Q. Preparation of amidoxime polyacrylonitrile chelating nanofibers and their application for adsorption of metal ions. Materials 2013, 6, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, X.; Sun, B.; Shen, M.; Tan, X.; Ding, Y.; Jiang, Z.; Wang, C. Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal. Chem. Eng. J. 2015, 268, 290–299. [Google Scholar] [CrossRef]
- Morillo Martín, D.; Faccini, M.; García, M.A.; Amantia, D. Highly efficient removal of heavy metal ions from polluted water using ion-selective polyacrylonitrile nanofibers. J. Environ. Chem. Eng. 2018, 6, 236–245. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, Y.G.; Yoon, Y.I.; Park, W.H. Hydrolysis of oxidized polyacrylonitrile nanofibrous webs and selective adsorption of harmful heavy metal ions. Polym. Degrad. Stab. 2017, 143, 207–213. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Yu, B.; Tan, Q.; Zhang, X.; Cong, H. Advanced Modified Polyacrylonitrile Membrane with Enhanced Adsorption Property for Heavy Metal Ions. Sci. Rep. 2018, 8, 1260. [Google Scholar] [CrossRef]
- Sole, K.C.; Hiskey, J.B. Solvent extraction of copper by Cyanex 272, Cyanex 302 and Cyanex 301. Hydrometallurgy 1995, 37, 129–147. [Google Scholar] [CrossRef]
- Saleh, M.I.; Bari, M.F.; Saad, B. Solvent extraction of lanthanum(III) from acidic nitrate-acetato medium by Cyanex 272 in toluene. Hydrometallurgy 2002, 63, 75–84. [Google Scholar] [CrossRef]
- Li, D. A review on yttrium solvent extraction chemistry and separation process. J. Rare Earths 2017, 35, 107–119. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.G.; Li, D.Q. Kinetics of extraction and stripping of Y(III) by cyanex 272 as an acidic extractant using a constant interfacial cell with laminar flow. Solvent Extr. Ion Exch. 2004, 22, 833–851. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Sunsandee, N.; Pancharoen, U.; Ramakul, P. Separation of yttrium from rare earth using hollow fiber-supported liquid membrane: Factorial design analysis. Desalin. Water Treat. 2016, 57, 3985–3994. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, S.; Li, D. Kinetics of ytterbium(III) extraction with Cyanex 272 using a constant interfacial cell with laminar flow. J. Alloy. Compd. 2006, 408, 1056–1060. [Google Scholar] [CrossRef]
- Das, S.; Behera, S.S.; Murmu, B.M.; Mohapatra, R.K.; Mandal, D.; Samantray, R.; Parhi, P.K.; Senanayake, G. Extraction of scandium(III) from acidic solutions using organo-phosphoric acid reagents: A comparative study. Sep. Purif. Technol. 2018, 202, 248–258. [Google Scholar] [CrossRef]
- Kazak, O.; Tor, A.; Akin, I.; Arslan, G. Preparation of new polysulfone capsules containing Cyanex 272 and their properties for Co(II) removal from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 1654–1661. [Google Scholar] [CrossRef]
- Basualto, C.; Gaete, J.; Molina, L.; Valenzuela, F.; Yañez, C.; Marco, J.F. Lanthanide sorbent based on magnetite nanoparticles functionalized with organophosphorus extractants. Sci. Technol. Adv. Mater. 2015, 16, 035010. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; John wiley & Sons Inc.: West Sussex, UK, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Almuhamed, S.; Bonne, M.; Khenoussi, N.; Brendle, J.; Schacher, L.; Lebeau, B.; Adolphe, D.C. Electrospinning composite nanofibers of polyacrylonitrile/synthetic Na-montmorillonite. J. Ind. Eng. Chem. 2016, 35, 146–152. [Google Scholar] [CrossRef]
- Neisiany, R.E.; Khorasani, S.N.; Kong Yoong Lee, J.; Ramakrishna, S. Encapsulation of epoxy and amine curing agent in PAN nanofibers by coaxial electrospinning for self-healing purposes. RSC Adv. 2016, 6, 70056–70063. [Google Scholar] [CrossRef]
- Tunsu, C.; Lapp, J.B.; Ekberg, C.; Retegan, T. Selective separation of yttrium and europium using Cyanex 572 for applications in fluorescent lamp waste processing. Hydrometallurgy 2016, 166, 98–106. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Kovalev, V.; Khozhaenko, E.; Khotimchenko, R. Removal of yttrium (III) ions from water solutions by alginate compounds. Int. J. Environ. Sci. Technol. 2015, 12, 3107–3116. [Google Scholar] [CrossRef] [Green Version]
- Prodromou, M.; Pashalidis, I. Europium adsorption by non-treated and chemically modified opuntia ficus indica cactus fibres in aqueous solutions. Desalin. Water Treat. 2016, 57, 5079–5088. [Google Scholar] [CrossRef]
- Dubey, S.S.; Grandhi, S. Sorption studies of yttrium (III) ions on surfaces of nano-thorium (IV) oxide and nano-zirconium(IV) oxide. Int. J. Environ. Sci. Technol. 2019, 16, 59–70. [Google Scholar] [CrossRef]
- Granados-Correa, F.; Jiménez-Reyes, M. Combustion synthesis of BaCO3 and its application for eu(III) adsorption from aqueous solution. Sep. Sci. Technol. 2011, 46, 2360–2366. [Google Scholar] [CrossRef]
- Dubey, S.S.; Grandhi, S. Sorption studies of yttrium (III) ions on nano maghemite. J. Environ. Chem. Eng. 2016, 4, 4719–4730. [Google Scholar] [CrossRef]
- Misaelides, P.; Sarri, S.; Zamboulis, D.; Gallios, G.; Zhuravlev, I.; Strelko, V.V. Separation of europium from aqueous solutions using Al3+- and Fe3+-doped zirconium and titanium phosphates. J. Radioanal. Nucl. Chem. 2006, 268, 53–58. [Google Scholar] [CrossRef]
- Quinn, K.A.; Byrne, R.H.; Schijf, J. Sorption of yttrium and rare earth elements by amorphous ferric hydroxide: Influence of temperature. Environ. Sci. Technol. 2007, 41, 541–546. [Google Scholar] [CrossRef]
- Quinn, K.A.; Byrne, R.H.; Schijf, J. Sorption of yttrium and rare earth elements by amorphous ferric hydroxide: Influence of solution complexation with carbonate. Geochim. Cosmochim. Acta 2006, 70, 4151–4165. [Google Scholar] [CrossRef]
- Kang, M.J.; Hahn, P.S. Adsorption behavior of aqueous europium on kaolinite under various disposal conditions. Korean J. Chem. Eng. 2004, 21, 419–424. [Google Scholar] [CrossRef]
- Synowczynski-Dunn, J.; Behler, K.; Marvel, C.; Harmer, M.; LaSalvia, J.C. First Principles Model of Yttrium Adsorption On Boron Suboxide (0001) Surface. In Proceedings of the 42nd International Conference on Advanced Ceramics and Composites: Ceramic Engineering and Science Proceedings, Daytona Beach, FL, USA, 21–26 January 2018; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; Volume 39, pp. 205–212. [Google Scholar]
- Vijayaraghavan, K.; Sathishkumar, M.; Balasubramanian, R. Biosorption of lanthanum, cerium, europium, and ytterbium by a brown marine alga, turbinaria conoides. Ind. Eng. Chem. Res. 2010, 49, 4405–4411. [Google Scholar] [CrossRef]
- Yu, B.; Feng, J.; Hu, Z.; Chi, R.; Zhou, F. Lanthanum (III) and Yttrium (III) Adsorption on Montmorillonite: The Role of Aluminum Ion in Solution and Minerals. Miner. Process. Extr. Metall. Rev. 2019, 1–10. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.C.; Guibal, E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Hayat, T.; Alharbi, N.S.; Chen, C. Adsorption of Europium on Al-substituted goethite. J. Mol. Liq. 2017, 236, 445–451. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, T.; Liu, H.; Xu, B.; Xie, J. Kinetics and thermodynamics of Eu(III) and U(VI) adsorption onto palygorskite. J. Mol. Liq. 2016, 219, 272–278. [Google Scholar] [CrossRef]
- Mohammedi, H.; Miloudi, H.; Tayeb, A.; Bertagnolli, C.; Boos, A. Study on the extraction of lanthanides by a mesoporous MCM-41 silica impregnated with Cyanex 272. Sep. Purif. Technol. 2019, 209, 359–367. [Google Scholar] [CrossRef]







| Y(III) | Eu(III) | ||||
|---|---|---|---|---|---|
| Adsorbent | qmax (mg/g) | Ref. | Adsorbent | qmax (mg/g) | Ref. |
| PAN-272 | 200 | This work | PAN-272 | 400 | This work |
| Alginate | 181.8 | [40] | Opuntia ficus indica cactus fibers | 72 | [41] |
| Nano-thorium(IV) oxide and nano-zirconium(IV)oxide | 10–18 | [42] | Barium carbonate | 16 | [43] |
| Maghemite | 13.5 | [44] | Zr and Ti phosphates | 20–50 | [45] |
| Ferric hydroxide | N/R | [46,47] | Kaolinite | 1.2 | [48] |
| Boron suboxide | N/R | [49] | T. Conoides (alga) | 138.2 | [50] |
| Montmorillonite | N/R | [51] | Chitosan microparticles | 375 | [52] |
| Al-substituted goethite | 6.75 | [53] | |||
| Palygorskite | 24.26 | [54] | |||
| Target Metal Ion | Langmuir Constants | Freundlich Constants | |||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | kL (L/mg) | R2 | qmax (mg/g) | kF | n | R2 | |
| Y(III) | 200 | 0.2564 | 1 | 380 | 17.697 | 2.24 | 0.8402 |
| Eu(III) | 400 | 0.0646 | 0.9879 | 396 | 92.2996 | 4.24989 | 0.6825 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morillo Martín, D.; Diaz Jalaff, L.; García, M.A.; Faccini, M. Selective Recovery of Europium and Yttrium Ions with Cyanex 272-Polyacrylonitrile Nanofibers. Nanomaterials 2019, 9, 1648. https://doi.org/10.3390/nano9121648
Morillo Martín D, Diaz Jalaff L, García MA, Faccini M. Selective Recovery of Europium and Yttrium Ions with Cyanex 272-Polyacrylonitrile Nanofibers. Nanomaterials. 2019; 9(12):1648. https://doi.org/10.3390/nano9121648
Chicago/Turabian StyleMorillo Martín, Diego, Leslie Diaz Jalaff, Maria A. García, and Mirko Faccini. 2019. "Selective Recovery of Europium and Yttrium Ions with Cyanex 272-Polyacrylonitrile Nanofibers" Nanomaterials 9, no. 12: 1648. https://doi.org/10.3390/nano9121648
APA StyleMorillo Martín, D., Diaz Jalaff, L., García, M. A., & Faccini, M. (2019). Selective Recovery of Europium and Yttrium Ions with Cyanex 272-Polyacrylonitrile Nanofibers. Nanomaterials, 9(12), 1648. https://doi.org/10.3390/nano9121648






