Surface Morphology-Dependent Functionality of Titanium Dioxide–Nickel Oxide Nanocomposite Semiconductors
Abstract
:1. Introduction
2. Materials and Methods
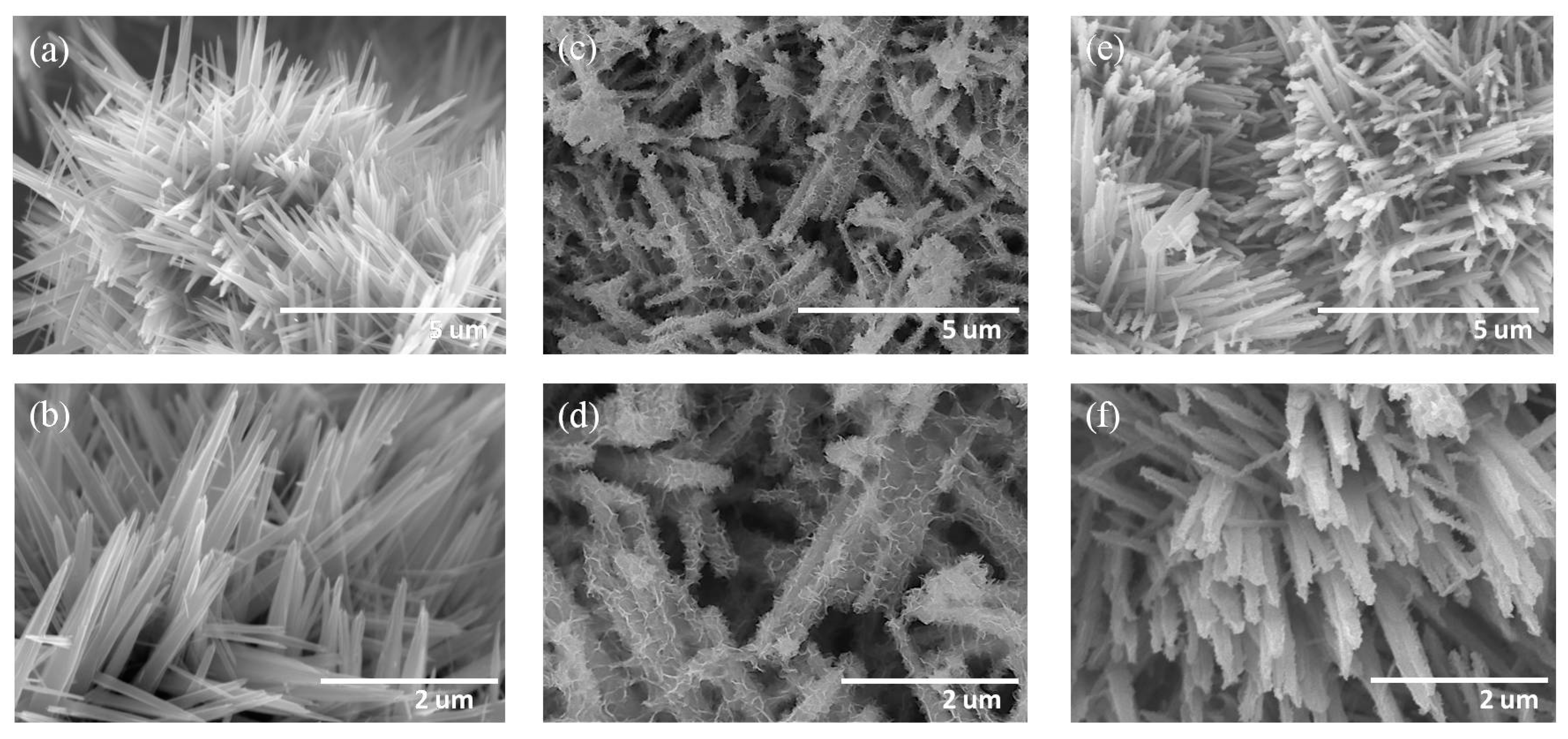
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luna-Flores, A.; Sosa-Sánchez, J.L.; Morales-Sánchez, M.A.; Agustín-Serrano, R.; Luna-López, J.A. An Easy-Made, Economical and Efficient Carbon-Doped Amorphous TiO2 Photocatalyst Obtained by Microwave Assisted Synthesis for the Degradation of Rhodamine B. Materials 2017, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Xu, N.C.; Wang, C.C.; Wei, D.H. Fabrication of Nanosized Island-Like CdO Crystallites-Decorated TiO2 Rod Nanocomposites via a Combinational Methodology and Their Low-Concentration NO2 Gas-Sensing Behavior. Materials 2017, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Wang, C.C. Hydrothermally derived zinc sulfide sphere-decorated titanium dioxide flower-like composites and their enhanced ethanol gas-sensing performance. J. Alloy Compd. 2018, 730, 333–341. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, M.; Qin, X. Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters. Nanomaterials 2019, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Ma, C.; Dai, B.; Yu, F. Enhanced Photocatalytic Degradation of Organic Dyes via Defect-Rich TiO2 Prepared by Dielectric Barrier Discharge Plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, Y.C. Design of Nanoscaled Surface Morphology of TiO2–Ag2O Composite Nanorods through Sputtering Decoration Process and Their Low-Concentration NO2 Gas-Sensing Behaviors. Nanomaterials 2019, 9, 1150. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y.; Han, J.; Guo, R.; Xiong, H.; Yin, Y. TiO2/NiO hybrid shells: P–n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A 2015, 3, 20727–20735. [Google Scholar] [CrossRef]
- Sun, G.J.; Kheel, H.; Park, S.; Lee, S.; Park, S.E.; Lee, C. Synthesis of TiO2 nanorods decorated with NiO nanoparticles and their acetone sensing properties. Ceram. Int. 2016, 42, 1063–1069. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Yang, J.; Wang, B.; Ma, M.; Xu, F.; Sun, R.; Zhang, X. Enhanced Photocatalytic Activity of CdS-Decorated TiO2/Carbon Core-Shell Microspheres Derived from Microcrystalline Cellulose. Materials 2016, 9, 245. [Google Scholar] [CrossRef]
- Ha, M.N.; Zhu, F.; Liu, Z.; Wang, L.; Liu, L.; Lu, G.; Zhao, Z. Morphology-controlled synthesis of SrTiO3/TiO2 heterostructures and their photocatalytic performance for water splitting. RSC Adv. 2016, 6, 21111–21118. [Google Scholar] [CrossRef]
- Wang, M.; Cui, L.; Li, S.; Li, Z.; Ma, T.; Luan, G.; Liu, W.; Zhang, F. Facile fabrication hybrids of TiO2@ZnO tubes with enhanced photocatalytic properties. RSC Adv. 2016, 6, 58452–58457. [Google Scholar] [CrossRef]
- Fujii, E.; Tomozawa, A.; Torii, H.; Takayama, R. Preferred Orientations of NiO Films Prepared by Plasma-Enhanced Metalorganic Chemical Vapor Deposition. Jpn. J. Appl. Phys. 1996, 35, L328–L330. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Haug, C. Enhanced photocatalytic degradation of an azo textile dye by using TiO2/NiO coupled nanoparticles: Optimization of synthesis and operational key factors. Mater. Sci. Semicond. Process. 2014, 27, 240–253. [Google Scholar] [CrossRef]
- Kaye, S.S.; Long, J.R. Hydrogen Storage in the Dehydrated Prussian Blue Analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 2005, 127, 6506–6507. [Google Scholar] [CrossRef]
- Gaspera, E.D.; Guglielmi, M.; Agnoli, S.; Granozzi, G.; Post, M.L.; Bello, V.; Mattei, G.; Martucci, A. Au Nanoparticles in Nanocrystalline TiO2-NiO Films for SPR-Based, Selective H2S Gas Sensing. Chem. Mater. 2010, 22, 3407–3417. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Dong, G.; Ma, L.; Ma, J. NiO/ZnO p–n heterostructures and their gas sensing properties for reduced operating temperature. RSC Adv. 2016, 6, 109091–109098. [Google Scholar] [CrossRef]
- Liang, K.; Tang, X.; Hu, W. High-performance three-dimensional nanoporous NiO film as a supercapacitor electrode. J. Mater. Chem. 2012, 22, 11062–11067. [Google Scholar] [CrossRef]
- Varghese, B.; Reddy, M.V.; Yanwu, Z.; Chang, S.L.; Hoong, T.C.; Rao, G.V.S.; Chowdari, B.V.R.; Wee, A.T.S.; Lim, C.T.; Sow, C.-H. Fabrication of NiO Nanowall Electrodes for High Performance Lithium Ion Battery. Chem. Mater. 2008, 20, 3360–3367. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, D.; Yao, S.; Liu, F.; Wang, B.; Sun, P.; Gao, Y.; Chuai, X.; Lu, G. Hierarchical core/shell ZnO/NiO nanoheterojunctions synthesized by ultrasonic spray pyrolysis and their gas-sensing performance. CrystEngComm 2016, 18, 8101–8107. [Google Scholar] [CrossRef]
- Wang, C.; Cui, X.; Liu, J.; Zhou, X.; Cheng, X.; Sun, P.; Hu, X.; Li, X.; Zheng, J.; Lu, G. Design of Superior Ethanol Gas Sensor Based on Al-Doped NiO Nanorod-Flowers. ACS Sens. 2016, 1, 131–136. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Abbasi, M.A.; Liu, X.; AlSalhi, M.S.; Willander, M. The Synthesis of NiO/TiO2Heterostructures and Their Valence Band Offset Determination. J. Nanomater. 2014, 6, 928658. [Google Scholar] [CrossRef]
- Lin, J.; Shen, J.; Wang, R.; Cui, J.; Zhou, W.; Hu, P.; Liu, D.; Liu, H.; Wang, J.; Boughton, R.I.; et al. Nano-p–n junctions on surface-coarsened TiO2nanobelts with enhanced photocatalytic activity. J. Mater. Chem. 2011, 21, 5106–5113. [Google Scholar] [CrossRef]
- Guo, J.; Fu, W.; Yang, H.; Yu, Q.; Zhao, W.; Zhou, X.; Sui, Y.; Ding, J.; Li, Y.; Cheng, S.; et al. A NiO/TiO2 junction electrode constructed using self-organized TiO2 nanotube arrays for highly efficient photoelectrocatalytic visible light activations. J. Phys. D Appl. Phys. 2010, 43, 245202. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. Band alignment investigations of heterostructure NiO/TiO2 nanomaterials used as efficient heterojunction earth-abundant metal oxide photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 19279–19288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Li, Y.; Sun, Q.; Wang, Y.; Wood, B.J.; Liu, P.; Yang, D.; Zhao, H. Vertically aligned nanorod-like rutile TiO2 single crystal nanowire bundles with superior electron transport and photoelectrocatalytic properties. J. Mater. Chem. 2012, 22, 2465–2472. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Gao, T.; Zhang, H.; Yu, H. NiO nanosheet/TiO2 nanorod-constructed p–n heterostructures for improved photocatalytic activity. Appl. Surf. Sci. 2016, 364, 322–331. [Google Scholar] [CrossRef]
- Lin, H.; Ye, H.; Chen, S.; Chen, Y. One-pot hydrothermal synthesis of BiPO4/BiVO4 with enhanced visible-light photocatalytic activities for methylene blue degradation. RSC Adv. 2014, 4, 10968–10974. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lung, T.W. Growth of Hydrothermally Derived CdS-Based Nanostructures with Various Crystal Features and Photoactivated Properties. Nanoscale Res. Lett. 2016, 11, 264. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lo, Y.R.; Wang, C.C.; Xu, N.C. Shell Layer Thickness-Dependent Photocatalytic Activity of Sputtering Synthesized Hexagonally Structured ZnO-ZnS Composite Nanorods. Materials 2018, 11, 87. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, Y.C.; Hung, C.S. Sputtering control of Ag2O decoration configurations on ZnO nanorods and their surface arrangement effects on photodegradation ability toward methyl orange. Nanotechnology 2019, 30, 495701–495711. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Chung, C.C.; Lin, T.Y.; Cheng, Y.R. Synthesis and microstructure-dependent photoactivated properties of three-dimensional cadmium sulfide crystals. J. Alloy Compd. 2016, 688, 769–775. [Google Scholar] [CrossRef]
- Liang, Y.C.; Cheng, Y.R.; Hsia, H.Y.; Chung, C.C. Fabrication and reducing gas detection characterization of highly-crystalline p-type zinc chromite oxide thin film. Appl. Surf. Sci. 2016, 364, 837–842. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, H.M.; Kim, T.H.; Chung, J.H.; Kang, Y.C.; Lee, J.H. Enhanced Ethanol Sensing Characteristics of In2O3-Decorated NiO Hollow Nanostructures via Modulation of Hole Accumulation Layers. ACS Appl. Mater. Interfaces 2014, 6, 18197–18204. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Hsia, H.Y.; Cheng, Y.R.; Lee, C.M.; Liu, S.L.; Lin, T.Y.; Chung, C.C. Crystalline quality-dependent gas detection behaviors of zinc oxide–zinc chromite p–n heterostructures. CrystEngComm 2015, 17, 4190–4199. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lo, Y.J. High-temperature solid-state reaction induced structure modifications and associated photoactivity and gas-sensing performance of binary oxide one-dimensional composite system. RSC Adv. 2017, 7, 29428–29439. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.J.; Kheel, H.; Choi, S.; Hyun, S.K.; Lee, C. Prominent Gas Sensing Performance of TiO2-Core/NiO-Shell Nanorod Sensors. J. Nanosci. Nanotechnol. 2017, 17, 4099–4102. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Tuantranont, A.; Comini, E.; Sberveglieri, G.; Wlodarski, W. Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Films 2009, 517, 2775–2780. [Google Scholar] [CrossRef]






| Materials | Operating Temperature | Acetone Concentration | Response (Ra/Rg or Rg/Ra) | Reference |
|---|---|---|---|---|
| NiO nanosheets–TiO2 flowers | 300 °C | 100 ppm | 51.6 (Rg/Ra) | (this work) |
| NiO nanoparticles–TiO2 flowers | 300 °C | 100 ppm | 13.54 (Rg/Ra) | (this work) |
| TiO2–NiO nanorod | 400 °C | 200 ppm | 9.81 (Rg/Ra) | [37] |
| TiO2–NiO nanorod | 400 °C | 200 ppm | 9.33 (Ra/Rg) | [8] |
| 95 wt% TiO2–5 wt% NiO composite film | 300 °C | 100 ppm | 6 (Rg/Ra) | [38] |
| 99 wt% TiO2–1 wt% NiO composite film | 400 °C | 100 ppm | 6.5 (Rg/Ra) | [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-C.; Xu, N.-C.; Chiang, K.-J. Surface Morphology-Dependent Functionality of Titanium Dioxide–Nickel Oxide Nanocomposite Semiconductors. Nanomaterials 2019, 9, 1651. https://doi.org/10.3390/nano9121651
Liang Y-C, Xu N-C, Chiang K-J. Surface Morphology-Dependent Functionality of Titanium Dioxide–Nickel Oxide Nanocomposite Semiconductors. Nanomaterials. 2019; 9(12):1651. https://doi.org/10.3390/nano9121651
Chicago/Turabian StyleLiang, Yuan-Chang, Nian-Cih Xu, and Kai-Jen Chiang. 2019. "Surface Morphology-Dependent Functionality of Titanium Dioxide–Nickel Oxide Nanocomposite Semiconductors" Nanomaterials 9, no. 12: 1651. https://doi.org/10.3390/nano9121651
APA StyleLiang, Y.-C., Xu, N.-C., & Chiang, K.-J. (2019). Surface Morphology-Dependent Functionality of Titanium Dioxide–Nickel Oxide Nanocomposite Semiconductors. Nanomaterials, 9(12), 1651. https://doi.org/10.3390/nano9121651





